当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Interactions of Water with the (111) and (100) Surfaces of Ceria
The Journal of Physical Chemistry C ( IF 3.7 ) Pub Date : 2017-09-22 00:00:00 , DOI: 10.1021/acs.jpcc.7b08150 Thomas Kropp 1 , Joachim Paier 1 , Joachim Sauer 1
The Journal of Physical Chemistry C ( IF 3.7 ) Pub Date : 2017-09-22 00:00:00 , DOI: 10.1021/acs.jpcc.7b08150 Thomas Kropp 1 , Joachim Paier 1 , Joachim Sauer 1
Affiliation
|
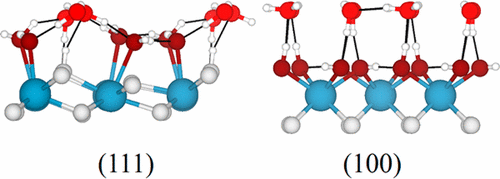
This density functional theory study compares water adsorption on the ceria (111) and (100) surfaces. In the absence of water, the (111) surface is more stable than the polar (100) surface, but at higher water coverages the (100) surface is favored due to the formation of a highly stable “square ice” layer on top of the fully hydroxylated surface. At ceria (111), an amorphous ice layer is favored that consists predominantly of molecularly adsorbed water. This change in the water–surface interactions stems from the different surface terminations: the lower coordination numbers of ions at the (100) surface lead to higher water adsorption energies and a preference toward dissociative adsorption. Furthermore, the distance between neighboring surface oxygen ions (274 pm) is very close to the O–O distance in bulk ice, which facilitates the growth of a well-ordered ice layer. At the (111) surface, the distance between neighboring surface oxygen ions is significantly larger (388 pm), which hampers water–water interactions in the surface layer.
中文翻译:

水与二氧化铈(111)和(100)表面的相互作用
该密度泛函理论研究比较了二氧化铈(111)和(100)表面上的水吸附。在没有水的情况下,(111)表面比极性(100)表面更稳定,但是在较高的水覆盖率下,由于(100)表面在冰盖的顶部形成了高度稳定的“方冰”层,因此受到青睐。完全羟基化的表面。在二氧化铈(111)处,主要由分子吸附的水组成的无定形冰层是有利的。水-表面相互作用的这种变化源于不同的表面末端:(100)表面上离子的较低配位数导致较高的水吸收能,并且倾向于解离吸附。此外,相邻表面氧离子之间的距离(274 pm)非常接近散装冰中的O–O距离,促进了井井有条的冰层的生长。在(111)表面,相邻表面氧离子之间的距离明显更大(388 pm),这阻碍了表面层中水与水的相互作用。
更新日期:2017-09-22
中文翻译:

水与二氧化铈(111)和(100)表面的相互作用
该密度泛函理论研究比较了二氧化铈(111)和(100)表面上的水吸附。在没有水的情况下,(111)表面比极性(100)表面更稳定,但是在较高的水覆盖率下,由于(100)表面在冰盖的顶部形成了高度稳定的“方冰”层,因此受到青睐。完全羟基化的表面。在二氧化铈(111)处,主要由分子吸附的水组成的无定形冰层是有利的。水-表面相互作用的这种变化源于不同的表面末端:(100)表面上离子的较低配位数导致较高的水吸收能,并且倾向于解离吸附。此外,相邻表面氧离子之间的距离(274 pm)非常接近散装冰中的O–O距离,促进了井井有条的冰层的生长。在(111)表面,相邻表面氧离子之间的距离明显更大(388 pm),这阻碍了表面层中水与水的相互作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号