当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Iron-Promoted Difunctionalization of Alkenes by Phenylselenylation/1,2-Aryl Migration
Organic Letters ( IF 4.9 ) Pub Date : 2017-09-22 00:00:00 , DOI: 10.1021/acs.orglett.7b02751 Ping Wu 1, 2 , Kaikai Wu 1 , Liandi Wang 1 , Zhengkun Yu 1, 3
Organic Letters ( IF 4.9 ) Pub Date : 2017-09-22 00:00:00 , DOI: 10.1021/acs.orglett.7b02751 Ping Wu 1, 2 , Kaikai Wu 1 , Liandi Wang 1 , Zhengkun Yu 1, 3
Affiliation
|
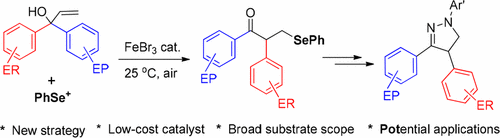
Iron-promoted difunctionalization of α,α-diaryl and α-aryl-α-alkyl allylic alcohols has been efficiently achieved by means of N-(phenylseleno)phthalimide (N-PSP) under mild conditions. An in situ generated phenylselenium cation (PhSe+) was added to the olefinic C═C bond to initiate the regioselective phenylselenylation with concomitant 1,2-aryl migration, following a migration preference contrary to the well-known radical pathway. Hydrazonation of the resultant alkene difunctionalization products, that is, α-aryl-β-phenylselenyl ketones, and subsequent copper-catalyzed dehydroselenylation efficiently afforded functionalized 2-pyrazoline derivatives.
中文翻译:

铁通过苯基硒基化/ 1,2-芳基迁移促进烯烃的双官能化
铁,α,α-二芳基和α-芳基-α-烷基烯丙基醇的双官能化已在温和的条件下通过N-(苯基硒代)邻苯二甲酰亚胺(N-PSP)有效地实现了。将原位生成的苯基硒阳离子(PhSe +)添加到烯烃的C═C键上,以一种与众所周知的自由基途径相反的迁移偏好,伴随着1,2-芳基迁移,引发区域选择性的苯基硒基化反应。所得到的烯烃双官能化产物,即α-芳基-β-苯基硒烯酮的氢解,以及随后的铜催化的脱氢硒烯化有效地提供了官能化的2-吡唑啉衍生物。
更新日期:2017-09-22
中文翻译:

铁通过苯基硒基化/ 1,2-芳基迁移促进烯烃的双官能化
铁,α,α-二芳基和α-芳基-α-烷基烯丙基醇的双官能化已在温和的条件下通过N-(苯基硒代)邻苯二甲酰亚胺(N-PSP)有效地实现了。将原位生成的苯基硒阳离子(PhSe +)添加到烯烃的C═C键上,以一种与众所周知的自由基途径相反的迁移偏好,伴随着1,2-芳基迁移,引发区域选择性的苯基硒基化反应。所得到的烯烃双官能化产物,即α-芳基-β-苯基硒烯酮的氢解,以及随后的铜催化的脱氢硒烯化有效地提供了官能化的2-吡唑啉衍生物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号