当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Phenoxazine: A Privileged Scaffold for Radical-Trapping Antioxidants
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2017-09-22 00:00:00 , DOI: 10.1021/acs.joc.7b02025 Luke A. Farmer 1 , Evan A. Haidasz 1 , Markus Griesser 1 , Derek A. Pratt 1
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2017-09-22 00:00:00 , DOI: 10.1021/acs.joc.7b02025 Luke A. Farmer 1 , Evan A. Haidasz 1 , Markus Griesser 1 , Derek A. Pratt 1
Affiliation
|
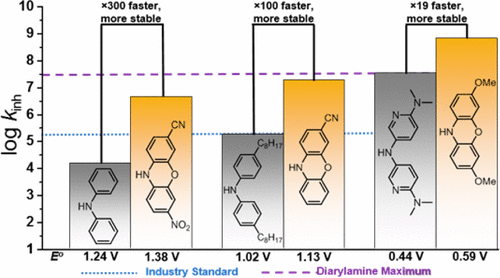
Diphenylamines are widely used to protect petroleum-derived products from autoxidation. Their efficacy as radical-trapping antioxidants (RTAs) relies on a balance of fast H-atom transfer kinetics and stability to one-electron oxidation by peroxidic species. Both H-atom transfer and one-electron oxidation are enhanced by substitution with electron-donating substituents, such as the S-atom in phenothiazines, another important class of RTA. Herein we report the results of our investigations of the RTA activity of the structurally related, but essentially ignored, phenoxazines. We find that the H-atom transfer reactivity of substituted phenoxazines follows an excellent Evans–Polanyi correlation spanning kinh = 4.5 × 106 M–1 s–1 and N–H BDE = 77.4 kcal mol–1 for 3-CN,7-NO2-phenoxazine to kinh = 6.6 × 108 M–1 s–1 and N–H BDE = 71.8 kcal mol–1 for 3,7-(OMe)2-phenoxazine (37 °C). The reactivity of the latter compound is the greatest of any RTA ever reported and is likely to represent a reaction without an enthalpic barrier since log A for this reaction is likely ∼8.5. The very high reactivity of most of the phenoxazines studied required the determination of their kinetic parameters by inhibited autoxidations in the presence of a very strong H-bonding cosolvent (DMSO), which slowed the observed rates by up to 2 orders of magnitude by dynamically reducing the equilibrium concentration of (free) phenoxazine as an H-atom donor. Despite their remarkably high reactivity toward peroxyl radicals, the phenoxazines were found to be comparatively stable to one-electron oxidation relative to diphenylamines and phenothiazines (E° ranging from 0.59 to 1.38 V vs NHE). Thus, phenoxazines with comparable oxidative stability to commonly used diphenylamine and phenothiazine RTAs had significantly greater reactivity (by up to 2 orders of magnitude). Computations suggest that this remarkable balance in H-atom transfer kinetics and stability to one-electron oxidation results from the ability of the bridging oxygen atom in phenoxazine to serve as both a π-electron donor to stabilize the aminyl radical and σ-electron acceptor to destabilize the aminyl radical cation. Perhaps most excitingly, phenoxazines have “non-classical” RTA activity, where they trap >2 peroxyl radicals each, at ambient temperatures.
中文翻译:

苯恶嗪:自由基捕获抗氧化剂的特权支架。
二苯胺广泛用于保护石油衍生产品免遭自氧化。它们作为自由基捕获抗氧化剂(RTA)的功效取决于快速H原子转移动力学和对过氧化物物种对单电子氧化的稳定性之间的平衡。H-原子的转移和单电子氧化都可以通过供电子性取代基(如吩噻嗪中的S-原子,另一类重要的RTA)进行取代来增强。本文中,我们报告了我们对结构相关但基本被忽略的吩恶嗪的RTA活性进行研究的结果。我们发现,取代的吩恶嗪的H原子转移反应性遵循极好的Evans-Polanyi相关性,范围为k inh = 4.5×10 6 M –1 s –1和3-CN,7-NO 2-吩恶嗪的N–H BDE = 77.4 kcal mol –1至k inh = 6.6×10 8 M –1 s –1和3 –––––– H BDE = 71.8 kcal mol –1。 7-(OMe)2-吩恶嗪(37°C)。后一种化合物的反应活性是有史以来报道的所有RTA中最大的,并且很可能代表没有对数屏障的反应,因为log A该反应的可能约为8.5。所研究的大多数吩恶嗪具有很高的反应活性,需要在非常强的H键合助溶剂(DMSO)的存在下通过抑制自氧化来确定其动力学参数,这会通过动态还原而将观察到的速率降低多达2个数量级。 (游离)吩恶嗪作为H原子供体的平衡浓度。尽管它们朝向过氧自由基非常高的反应性,发现吩恶嗪是相对稳定的氧化相对于二苯胺和吩噻嗪单电子(É°相对于NHE为0.59至1.38 V. 因此,具有与常用的二苯胺和吩噻嗪RTA相当的氧化稳定性的吩恶嗪具有显着更高的反应性(最高可达2个数量级)。计算表明,H原子转移动力学和对单电子氧化的稳定性之间的显着平衡是由于吩恶嗪中的桥连氧原子既充当π电子供体来稳定氨基自由基又是σ电子受体的能力而导致的。使氨基自由基阳离子不稳定。也许最令人兴奋的是,吩恶嗪具有“非经典”的RTA活性,在室温下它们各自捕获> 2个过氧自由基。
更新日期:2017-09-22
中文翻译:

苯恶嗪:自由基捕获抗氧化剂的特权支架。
二苯胺广泛用于保护石油衍生产品免遭自氧化。它们作为自由基捕获抗氧化剂(RTA)的功效取决于快速H原子转移动力学和对过氧化物物种对单电子氧化的稳定性之间的平衡。H-原子的转移和单电子氧化都可以通过供电子性取代基(如吩噻嗪中的S-原子,另一类重要的RTA)进行取代来增强。本文中,我们报告了我们对结构相关但基本被忽略的吩恶嗪的RTA活性进行研究的结果。我们发现,取代的吩恶嗪的H原子转移反应性遵循极好的Evans-Polanyi相关性,范围为k inh = 4.5×10 6 M –1 s –1和3-CN,7-NO 2-吩恶嗪的N–H BDE = 77.4 kcal mol –1至k inh = 6.6×10 8 M –1 s –1和3 –––––– H BDE = 71.8 kcal mol –1。 7-(OMe)2-吩恶嗪(37°C)。后一种化合物的反应活性是有史以来报道的所有RTA中最大的,并且很可能代表没有对数屏障的反应,因为log A该反应的可能约为8.5。所研究的大多数吩恶嗪具有很高的反应活性,需要在非常强的H键合助溶剂(DMSO)的存在下通过抑制自氧化来确定其动力学参数,这会通过动态还原而将观察到的速率降低多达2个数量级。 (游离)吩恶嗪作为H原子供体的平衡浓度。尽管它们朝向过氧自由基非常高的反应性,发现吩恶嗪是相对稳定的氧化相对于二苯胺和吩噻嗪单电子(É°相对于NHE为0.59至1.38 V. 因此,具有与常用的二苯胺和吩噻嗪RTA相当的氧化稳定性的吩恶嗪具有显着更高的反应性(最高可达2个数量级)。计算表明,H原子转移动力学和对单电子氧化的稳定性之间的显着平衡是由于吩恶嗪中的桥连氧原子既充当π电子供体来稳定氨基自由基又是σ电子受体的能力而导致的。使氨基自由基阳离子不稳定。也许最令人兴奋的是,吩恶嗪具有“非经典”的RTA活性,在室温下它们各自捕获> 2个过氧自由基。



























 京公网安备 11010802027423号
京公网安备 11010802027423号