当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalytic C(sp3)–H Alkylation via an Iron Carbene Intermediate
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-09-22 00:00:00 , DOI: 10.1021/jacs.7b07602 Jennifer R. Griffin 1 , Chloe I. Wendell 1 , Jacob A. Garwin 1 , M. Christina White 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-09-22 00:00:00 , DOI: 10.1021/jacs.7b07602 Jennifer R. Griffin 1 , Chloe I. Wendell 1 , Jacob A. Garwin 1 , M. Christina White 1
Affiliation
|
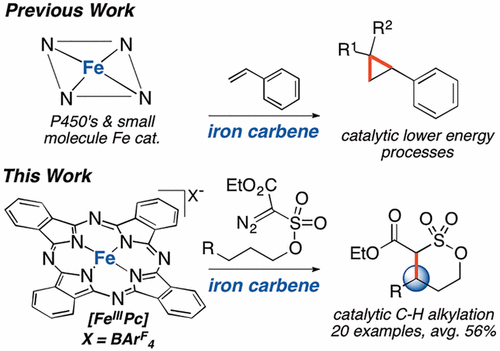
The catalytic transformation of a C(sp3)–H bond to a C(sp3)–C bond via an iron carbene intermediate represents a long-standing challenge. Despite the success of enzymatic and small molecule iron catalysts mediating challenging C(sp3)–H oxidations and aminations via high-valent iron oxos and nitrenes, C(sp3)–H alkylations via isoelectronic iron carbene intermediates have thus far been unsuccessful. Iron carbenes have been inert, or shown to favor olefin cyclopropanation and heteroatom-hydrogen insertion. Herein we report an iron phthalocyanine-catalyzed alkylation of allylic and benzylic C(sp3)–H bonds. Mechanistic investigations support that an electrophilic iron carbene mediates homolytic C–H cleavage and rebounds from the resulting organoiron intermediate to form the C–C bond; both steps are tunable via catalyst modifications. These studies suggest that for iron carbenes, distinct from other late metal carbenes, C–H cleavage is partially rate-determining and must be promoted to effect reactivity.
中文翻译:

经由铁碳中间体催化的C(sp 3)–H烷基化
通过卡宾铁中间体将C(sp 3)-H键催化转化为C(sp 3)-C键代表了长期存在的挑战。尽管酶和小分子铁催化剂成功地通过高价铁氧代和腈介导了具有挑战性的C(sp 3)-H氧化和胺化反应,但通过等电子卡宾中间体进行的C(sp 3)-H烷基化迄今仍未成功。卡宾铁是惰性的,或已证明有利于烯烃环丙烷化和杂原子-氢的插入。在这里我们报告铁酞菁催化烯丙基和苄基C(sp 3的烷基化)– H键。机理研究支持亲电性铁卡宾介导均相C–H裂解并从所得的有机铁中间体反弹形成C–C键;这两个步骤都可以通过修改催化剂来调整。这些研究表明,对于铁卡宾,不同于其他后期金属卡宾,C–H裂解是部分决定速率的反应,必须促进其反应性。
更新日期:2017-09-22
中文翻译:

经由铁碳中间体催化的C(sp 3)–H烷基化
通过卡宾铁中间体将C(sp 3)-H键催化转化为C(sp 3)-C键代表了长期存在的挑战。尽管酶和小分子铁催化剂成功地通过高价铁氧代和腈介导了具有挑战性的C(sp 3)-H氧化和胺化反应,但通过等电子卡宾中间体进行的C(sp 3)-H烷基化迄今仍未成功。卡宾铁是惰性的,或已证明有利于烯烃环丙烷化和杂原子-氢的插入。在这里我们报告铁酞菁催化烯丙基和苄基C(sp 3的烷基化)– H键。机理研究支持亲电性铁卡宾介导均相C–H裂解并从所得的有机铁中间体反弹形成C–C键;这两个步骤都可以通过修改催化剂来调整。这些研究表明,对于铁卡宾,不同于其他后期金属卡宾,C–H裂解是部分决定速率的反应,必须促进其反应性。









































 京公网安备 11010802027423号
京公网安备 11010802027423号