当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The trimerization of acetylenes involves a cascade of biradical and pericyclic processes
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2017-09-18 00:00:00 , DOI: 10.1039/c7ob01885a Gavin O. Jones 1, 2, 3 , Zachary J. Krebs 3, 4, 5, 6
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2017-09-18 00:00:00 , DOI: 10.1039/c7ob01885a Gavin O. Jones 1, 2, 3 , Zachary J. Krebs 3, 4, 5, 6
Affiliation
|
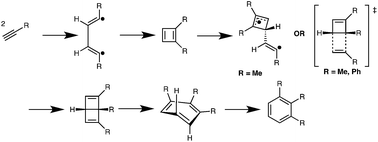
Thorough computational studies were performed on mechanisms and energies for the thermal trimerizations of neutral or electron-rich acetylenes used as cross-linkers in organic hard-masks for lithography applications. These studies indicate that the operative mechanism proceeds through initial cyclobutadiene formation via a biradical mechanism. Cyclobutadienes form Dewar benzenes via Diels–Alder cycloadditions, or biradical processes, or both, before producing benzenes by electrocyclic ring-opening reactions. These pathways are preferred to alternatives involving concerted trimerizations or mechanisms involving carbene intermediates.
中文翻译:

乙炔的三聚涉及双自由基和周环过程的级联
对用于光刻应用的有机硬掩模中用作交联剂的中性或富电子乙炔的热三聚的机理和能量进行了全面的计算研究。这些研究表明,其操作机理是通过双自由基机理通过最初的环丁二烯形成而进行的。在通过电环开环反应生产苯之前,环丁二烯通过Diels-Alder环加成或双自由基过程或两者形成杜瓦苯。这些途径比涉及协同三聚作用的替代方法或涉及卡宾中间体的机理更优选。
更新日期:2017-09-22
中文翻译:

乙炔的三聚涉及双自由基和周环过程的级联
对用于光刻应用的有机硬掩模中用作交联剂的中性或富电子乙炔的热三聚的机理和能量进行了全面的计算研究。这些研究表明,其操作机理是通过双自由基机理通过最初的环丁二烯形成而进行的。在通过电环开环反应生产苯之前,环丁二烯通过Diels-Alder环加成或双自由基过程或两者形成杜瓦苯。这些途径比涉及协同三聚作用的替代方法或涉及卡宾中间体的机理更优选。










































 京公网安备 11010802027423号
京公网安备 11010802027423号