当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Photochemical generation and trapping of 3-oxacyclohexyne
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2017-09-15 00:00:00 , DOI: 10.1039/c7ob01697b Rui Fan 1, 2, 3, 4 , Yuewei Wen 1, 2, 3, 4 , Dasan M. Thamattoor 1, 2, 3, 4
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2017-09-15 00:00:00 , DOI: 10.1039/c7ob01697b Rui Fan 1, 2, 3, 4 , Yuewei Wen 1, 2, 3, 4 , Dasan M. Thamattoor 1, 2, 3, 4
Affiliation
|
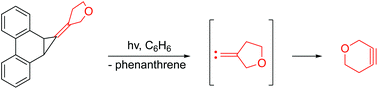
The strained heterocyclic alkyne, 3-oxacyclohexyne, was generated photochemically for the first time using a cyclopropanated phenanthrene precursor, and trapped by cyclopentadienones as Diels–Alder adducts. The precursor initially produced the putative 3-oxacyclopentylidenecarbene that subsequently rearranged to the cycloalkyne. Computational studies indicate that the carbene favors a singlet state, and the barrier for its ring expansion by a 1,2-shift of the carbon proximal to oxygen is lower in energy than the corresponding shift of the distal carbon.
中文翻译:

3-氧杂环己炔的光化学生成和捕获
首次使用环丙烷化的菲前体,通过光化学方法生成了应变杂环杂环炔基3-氧杂环己炔,并以环戊二烯酮的形式作为Diels-Alder加合物捕获。该前体最初产生了推定的3-氧杂环戊叉碳烯,随后将其重排成环炔烃。计算研究表明,卡宾偏向单线态,并且通过与近端碳的相应位移相比,通过碳的1,2-位移使它的环膨胀的壁垒的能量较低。
更新日期:2017-09-22
中文翻译:

3-氧杂环己炔的光化学生成和捕获
首次使用环丙烷化的菲前体,通过光化学方法生成了应变杂环杂环炔基3-氧杂环己炔,并以环戊二烯酮的形式作为Diels-Alder加合物捕获。该前体最初产生了推定的3-氧杂环戊叉碳烯,随后将其重排成环炔烃。计算研究表明,卡宾偏向单线态,并且通过与近端碳的相应位移相比,通过碳的1,2-位移使它的环膨胀的壁垒的能量较低。







































 京公网安备 11010802027423号
京公网安备 11010802027423号