当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Enantioselective aminocatalytic synthesis of tetrahydropyrano[2,3-c]pyrazoles via a domino Michael-hemiacetalization reaction with alkylidene pyrazolones
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2017-09-13 00:00:00 , DOI: 10.1039/c7ob02170d Rajendra Maity 1, 2, 3 , Subhas Chandra Pan 1, 2, 3
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2017-09-13 00:00:00 , DOI: 10.1039/c7ob02170d Rajendra Maity 1, 2, 3 , Subhas Chandra Pan 1, 2, 3
Affiliation
|
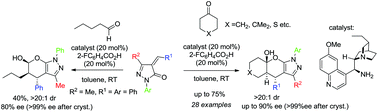
An enantioselective organocatalytic domino Michael-hemiacetalization reaction between alkylidene pyrazolones and cyclic ketones/pentanal has been revealed. The fused tetrahydropyranopyrazole products having three contiguous stereocentres were obtained with perfect diastereoselectivities and in moderate to good yields with good to high enantioselectivities. Also, few synthetic transformations of the product including the formation of a spiro derivative have been demonstrated.
中文翻译:

通过亚烷基吡唑啉酮的多米诺-迈克尔-半缩醛化反应对氨基选择性合成四氢吡喃并[2,3- c ]吡唑
已揭示了亚烷基吡唑啉酮与环酮/戊醛之间的对映选择性有机催化多米诺迈克尔-半缩醛化反应。获得具有三个连续立体中心的稠合四氢吡喃并吡唑产物,其具有非对映异构体完美的选择性,并以中等至良好的产率以及良好至高的对映选择性。同样,已经证明了很少的产物的合成转化,包括螺衍生物的形成。
更新日期:2017-09-22
中文翻译:

通过亚烷基吡唑啉酮的多米诺-迈克尔-半缩醛化反应对氨基选择性合成四氢吡喃并[2,3- c ]吡唑
已揭示了亚烷基吡唑啉酮与环酮/戊醛之间的对映选择性有机催化多米诺迈克尔-半缩醛化反应。获得具有三个连续立体中心的稠合四氢吡喃并吡唑产物,其具有非对映异构体完美的选择性,并以中等至良好的产率以及良好至高的对映选择性。同样,已经证明了很少的产物的合成转化,包括螺衍生物的形成。



























 京公网安备 11010802027423号
京公网安备 11010802027423号