当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Proton conduction in alkali metal ion-exchanged porous ionic crystals
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2017-09-06 00:00:00 , DOI: 10.1039/c7cp04619g Sayaka Uchida 1, 2, 3, 4, 5 , Reina Hosono 1, 2, 3, 4, 5 , Ryo Eguchi 3, 5, 6, 7, 8 , Ryosuke Kawahara 1, 2, 3, 4, 5 , Ryota Osuga 5, 9, 10, 11, 12 , Junko N. Kondo 5, 9, 10, 11, 12 , Mitsuhiro Hibino 3, 5, 6, 7, 8 , Noritaka Mizuno 3, 5, 6, 7, 8
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2017-09-06 00:00:00 , DOI: 10.1039/c7cp04619g Sayaka Uchida 1, 2, 3, 4, 5 , Reina Hosono 1, 2, 3, 4, 5 , Ryo Eguchi 3, 5, 6, 7, 8 , Ryosuke Kawahara 1, 2, 3, 4, 5 , Ryota Osuga 5, 9, 10, 11, 12 , Junko N. Kondo 5, 9, 10, 11, 12 , Mitsuhiro Hibino 3, 5, 6, 7, 8 , Noritaka Mizuno 3, 5, 6, 7, 8
Affiliation
|
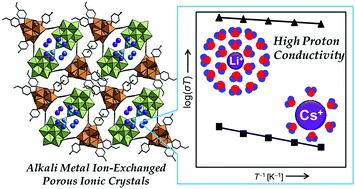
Proton conduction in alkali metal ion-exchanged porous ionic crystals A2[Cr3O(OOCH)6(etpy)3]2[α-SiW12O40]·nH2O [I-A+] (A = Li, Na, K, Cs, etpy = 4-ethylpyridine) is investigated. Single crystal and powder X-ray diffraction measurements show that I-A+ possesses analogous one-dimensional channels where alkali metal ions (A+) and water of crystallization exist. Impedance spectroscopy and water diffusion measurements of I-A+ show that proton conductivities are low (10−7–10−6 S cm−1) under low relative humidity (RH), and protons mostly migrate as H3O+ with H2O as vehicles (vehicle mechanism). The proton conductivity of I-A+ increases with the increase in RH and is largely dependent on the types of alkali metal ions. I-Li+ shows a high proton conductivity of 1.9 × 10−3 S cm−1 (323 K) and a low activation energy of 0.23 eV under RH 95%. Under high RH, alkali metal ions with high ionic potentials (e.g., Li+) form a dense and extensive hydrogen-bonding network of water molecules with mobile protons at the periphery, which leads to high proton conductivities and low activation energies via rearrangement of the hydrogen-bonding network (Grotthuss mechanism).
中文翻译:

碱金属离子交换的多孔离子晶体中的质子传导
质子传导在碱金属离子交换的多孔的离子晶体甲2 [CR 3 O(OOCH)6(etpy)3 ] 2 [α-硅钨酸12 ö 40 ]· Ñ ħ 2 -O [IA + ](A =锂,钠,K,Cs,etpy = 4-乙基吡啶)被研究。单晶和粉末X射线衍射测量表明,IA +具有类似的一维通道,其中存在碱金属离子(A +)和结晶水。IA +的阻抗谱和水扩散测量表明,质子电导率很低(10 -7 –10在相对湿度(RH)低的情况下为-6 S cm -1),并且质子大部分以H 3 O +的形式迁移,以H 2 O为媒介(车辆机理)。IA +的质子电导率随RH的增加而增加,并且在很大程度上取决于碱金属离子的类型。I-Li +在RH 95%的条件下显示出1.9×10 -3 S cm -1(323 K)的高质子传导率和0.23 eV的低活化能。在高RH下,具有高离子电势的碱金属离子(例如Li +)形成一个密集且广泛的水分子氢键网络,其外围带有可移动的质子,通过氢键网络的重排(格罗特斯机制),可导致高质子电导率和低活化能。
更新日期:2017-09-22
中文翻译:

碱金属离子交换的多孔离子晶体中的质子传导
质子传导在碱金属离子交换的多孔的离子晶体甲2 [CR 3 O(OOCH)6(etpy)3 ] 2 [α-硅钨酸12 ö 40 ]· Ñ ħ 2 -O [IA + ](A =锂,钠,K,Cs,etpy = 4-乙基吡啶)被研究。单晶和粉末X射线衍射测量表明,IA +具有类似的一维通道,其中存在碱金属离子(A +)和结晶水。IA +的阻抗谱和水扩散测量表明,质子电导率很低(10 -7 –10在相对湿度(RH)低的情况下为-6 S cm -1),并且质子大部分以H 3 O +的形式迁移,以H 2 O为媒介(车辆机理)。IA +的质子电导率随RH的增加而增加,并且在很大程度上取决于碱金属离子的类型。I-Li +在RH 95%的条件下显示出1.9×10 -3 S cm -1(323 K)的高质子传导率和0.23 eV的低活化能。在高RH下,具有高离子电势的碱金属离子(例如Li +)形成一个密集且广泛的水分子氢键网络,其外围带有可移动的质子,通过氢键网络的重排(格罗特斯机制),可导致高质子电导率和低活化能。











































 京公网安备 11010802027423号
京公网安备 11010802027423号