当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Achieving enhanced cell penetration of short conformationally constrained peptides through amphiphilicity tuning
Chemical Science ( IF 7.6 ) Pub Date : 2017-09-13 00:00:00 , DOI: 10.1039/c7sc03614k Yuan Tian 1, 2, 3, 4, 5 , Xiangze Zeng 6, 7, 8, 9, 10 , Jingxu Li 1, 2, 3, 4 , Yanhong Jiang 1, 2, 3, 4 , Hui Zhao 1, 2, 3, 4 , Dongyuan Wang 1, 2, 3, 4 , Xuhui Huang 6, 7, 8, 9, 10 , Zigang Li 1, 2, 3, 4
Chemical Science ( IF 7.6 ) Pub Date : 2017-09-13 00:00:00 , DOI: 10.1039/c7sc03614k Yuan Tian 1, 2, 3, 4, 5 , Xiangze Zeng 6, 7, 8, 9, 10 , Jingxu Li 1, 2, 3, 4 , Yanhong Jiang 1, 2, 3, 4 , Hui Zhao 1, 2, 3, 4 , Dongyuan Wang 1, 2, 3, 4 , Xuhui Huang 6, 7, 8, 9, 10 , Zigang Li 1, 2, 3, 4
Affiliation
|
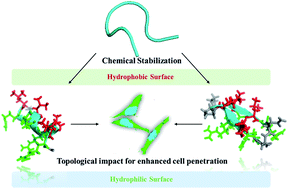
Due to their enhanced stability and cell permeability, cyclic cell-penetrating peptides have been widely used as delivery vectors for transporting cell-impermeable cargos into cells. In this study, we synthesized a panel of conformationally constrained peptides with either α-helix or β-hairpin conformations. We tuned the amphiphilicity of these constrained peptides with different distributions of charged or hydrophobic residues and compared their cellular uptake efficiencies in different cell lines. We found that the amphipathicity of these conformationally constrained peptides correlates well with their cellular uptake efficiency. We proposed that peptides with larger hydrophobic moments (HMs) have stronger binding affinities with the cell membrane which further accelerates the endocytosis process. This finding should provide an approach towards the design of more potent conformationally constrained cell-penetrating peptides for biomedical applications.
中文翻译:

通过两亲性调节实现短构象受限肽的增强细胞渗透
由于其增强的稳定性和细胞渗透性,环状穿透细胞的肽已被广泛用作将细胞不可渗透的货物运输到细胞中的递送载体。在这项研究中,我们合成了一组具有α-螺旋或β-发夹构象的构象受限肽。我们用不同的带电或疏水残基分布对这些受约束肽的两亲性进行了调整,并比较了它们在不同细胞系中的细胞吸收效率。我们发现这些构象受约束的肽的两亲性与其细胞摄取效率良好相关。我们提出具有较大疏水矩(HMs)的肽与细胞膜具有更强的结合亲和力,从而进一步加速了内吞过程。
更新日期:2017-09-22
中文翻译:

通过两亲性调节实现短构象受限肽的增强细胞渗透
由于其增强的稳定性和细胞渗透性,环状穿透细胞的肽已被广泛用作将细胞不可渗透的货物运输到细胞中的递送载体。在这项研究中,我们合成了一组具有α-螺旋或β-发夹构象的构象受限肽。我们用不同的带电或疏水残基分布对这些受约束肽的两亲性进行了调整,并比较了它们在不同细胞系中的细胞吸收效率。我们发现这些构象受约束的肽的两亲性与其细胞摄取效率良好相关。我们提出具有较大疏水矩(HMs)的肽与细胞膜具有更强的结合亲和力,从而进一步加速了内吞过程。











































 京公网安备 11010802027423号
京公网安备 11010802027423号