当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Diblock Terpolymers Are Tunable and pH Responsive Vehicles To Increase Hydrophobic Drug Solubility for Oral Administration
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2017-09-22 00:00:00 , DOI: 10.1021/acs.molpharmaceut.7b00458 Swapnil Tale 1 , Anatolii A. Purchel 1 , Molly C. Dalsin 1 , Theresa M. Reineke 1
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2017-09-22 00:00:00 , DOI: 10.1021/acs.molpharmaceut.7b00458 Swapnil Tale 1 , Anatolii A. Purchel 1 , Molly C. Dalsin 1 , Theresa M. Reineke 1
Affiliation
|
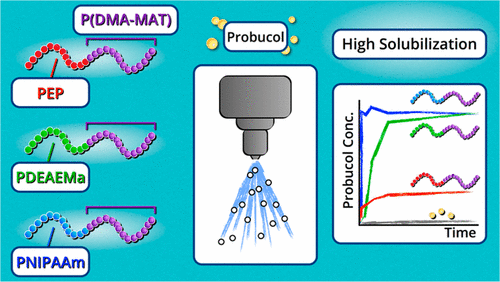
Synthetic polymers offer tunable platforms to create new oral drug delivery vehicles (excipients) to increase solubility, supersaturation maintenance, and bioavailability of poorly aqueous soluble pharmaceutical candidates. Five well-defined diblock terpolymers were synthesized via reversible addition–fragmentation chain transfer polymerization (RAFT) and consist of a first block of either poly(ethylene-alt-propylene) (PEP), poly(N-isopropylacrylamide) (PNIPAm), or poly(N,N-diethylaminoethyl methacrylate) (PDEAEMA) and a second hydrophilic block consisting of a gradient copolymer of N,N-dimethylacrylamide (DMA) and 2-methacrylamidotrehalose (MAT). This family of diblock terpolymers offers hydrophobic, hydrophilic, or H-bonding functionalities to serve as noncovalent sites of drug binding. Drug–polymer spray dried dispersions (SDDs) were created with a model drug, probucol, and characterized by differential scanning calorimetry (DSC). These studies revealed that probucol crystallinity decreased with increasing H-bonding sites available in the polymer. The PNIPAm-b-P(DMA-grad-MAT) systems revealed the best performance at pH 6.5, where immediate probucol release and effective maintenance of 100% supersaturation was found, which is important for facilitating drug solubility in more neutral conditions (intestinal environment). However, the PDEAEMA-b-P(DMA-grad-MAT) system revealed poor probucol dissolution at pH 6.5 and 5.1. Alternatively, at an acidic pH of 3.1, a rapid and high dissolution profile and effective supersaturation maintenance of up to 90% of the drug was found, which could be useful for triggering drug release in acidic environments (stomach). The PEP-b-P(DMA-grad-MAT) system showed poor performance (only ∼20% of drug solubility at pH 6.5), which was attributed to the low solubility of the polymers in the dissolution media. This work demonstrates the utility of diblock terpolymers as a potential new excipient platform to optimize design parameters for triggered release and solubilizing hydrophobic drug candidates for oral delivery.
中文翻译:

双嵌段三元共聚物是可调节的且具有pH响应性的载体,可提高口服给药的疏水性药物溶解度
合成聚合物提供可调平台,以创建新的口服药物输送媒介(赋形剂),以增加水溶性差的候选药物的溶解度,过饱和度维持率和生物利用度。五良好定义的二嵌段三元共聚物中经由可逆加成-断裂链转移聚合(RAFT)合成,并且由任一聚(乙烯的第一块的ALT -丙烯)(PEP),聚(Ñ -isopropylacrylamide)(PNIPAM),或聚(N,N-甲基丙烯酸二乙氨基乙酯)(PDEAEMA)和由N,N的梯度共聚物组成的第二亲水嵌段-二甲基丙烯酰胺(DMA)和2-甲基丙烯酰胺基海藻糖(MAT)。该二嵌段三元共聚物家族提供疏水,亲水或H键合功能,可作为药物结合的非共价位点。药物-聚合物喷雾干燥分散液(SDD)是使用普罗布考模型药物制成的,并通过差示扫描量热法(DSC)进行了表征。这些研究表明,普罗布考结晶度随聚合物中可用的H键合位点的增加而降低。PNIPAm- b -P(DMA- grad -MAT)系统在pH 6.5时表现出最佳性能,其中普罗布考立即释放并有效维持100%过饱和,这对于在更中性的条件下(肠道环境)促进药物溶解度非常重要)。但是,PDEAEMA- b-P(DMA- grad -MAT)系统显示在6.5和5.1的条件下普罗布考溶解较差。可替代地,在3.1的酸性pH下,发现快速且高的溶出曲线和高达90%的药物的有效过饱和维持,这对于触发在酸性环境(胃)中的药物释放可能是有用的。所述PEP- b -P(DMA-研究所-MAT)系统显示性能差(仅〜20%,在pH 6.5的药物溶解度的),这是由于在溶解介质中的聚合物的溶解度低。这项工作证明了二嵌段三元共聚物作为潜在的新型赋形剂平台的效用,可以优化触发释放的设计参数并增溶用于口服的疏水性候选药物。
更新日期:2017-09-22
中文翻译:

双嵌段三元共聚物是可调节的且具有pH响应性的载体,可提高口服给药的疏水性药物溶解度
合成聚合物提供可调平台,以创建新的口服药物输送媒介(赋形剂),以增加水溶性差的候选药物的溶解度,过饱和度维持率和生物利用度。五良好定义的二嵌段三元共聚物中经由可逆加成-断裂链转移聚合(RAFT)合成,并且由任一聚(乙烯的第一块的ALT -丙烯)(PEP),聚(Ñ -isopropylacrylamide)(PNIPAM),或聚(N,N-甲基丙烯酸二乙氨基乙酯)(PDEAEMA)和由N,N的梯度共聚物组成的第二亲水嵌段-二甲基丙烯酰胺(DMA)和2-甲基丙烯酰胺基海藻糖(MAT)。该二嵌段三元共聚物家族提供疏水,亲水或H键合功能,可作为药物结合的非共价位点。药物-聚合物喷雾干燥分散液(SDD)是使用普罗布考模型药物制成的,并通过差示扫描量热法(DSC)进行了表征。这些研究表明,普罗布考结晶度随聚合物中可用的H键合位点的增加而降低。PNIPAm- b -P(DMA- grad -MAT)系统在pH 6.5时表现出最佳性能,其中普罗布考立即释放并有效维持100%过饱和,这对于在更中性的条件下(肠道环境)促进药物溶解度非常重要)。但是,PDEAEMA- b-P(DMA- grad -MAT)系统显示在6.5和5.1的条件下普罗布考溶解较差。可替代地,在3.1的酸性pH下,发现快速且高的溶出曲线和高达90%的药物的有效过饱和维持,这对于触发在酸性环境(胃)中的药物释放可能是有用的。所述PEP- b -P(DMA-研究所-MAT)系统显示性能差(仅〜20%,在pH 6.5的药物溶解度的),这是由于在溶解介质中的聚合物的溶解度低。这项工作证明了二嵌段三元共聚物作为潜在的新型赋形剂平台的效用,可以优化触发释放的设计参数并增溶用于口服的疏水性候选药物。







































 京公网安备 11010802027423号
京公网安备 11010802027423号