当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Methanol on Anatase TiO2 (101): Mechanistic Insights into Photocatalysis
ACS Catalysis ( IF 11.3 ) Pub Date : 2017-09-21 00:00:00 , DOI: 10.1021/acscatal.7b02003 Martin Setvin 1 , Xiao Shi 2 , Jan Hulva 1 , Thomas Simschitz 1 , Gareth S. Parkinson 1 , Michael Schmid 1 , Cristiana Di Valentin 3 , Annabella Selloni 2 , Ulrike Diebold 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2017-09-21 00:00:00 , DOI: 10.1021/acscatal.7b02003 Martin Setvin 1 , Xiao Shi 2 , Jan Hulva 1 , Thomas Simschitz 1 , Gareth S. Parkinson 1 , Michael Schmid 1 , Cristiana Di Valentin 3 , Annabella Selloni 2 , Ulrike Diebold 1
Affiliation
|
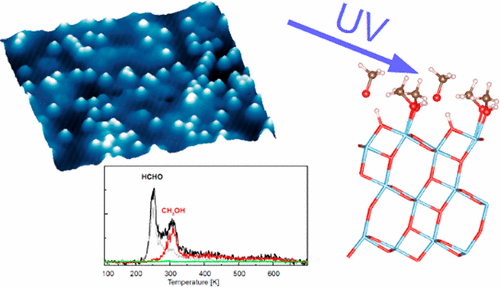
The photoactivity of methanol adsorbed on the anatase TiO2 (101) surface was studied by a combination of scanning tunneling microscopy (STM), temperature-programmed desorption (TPD), X-ray photoemission spectroscopy (XPS), and density functional theory (DFT) calculations. Isolated methanol molecules adsorbed at the anatase (101) surface show a negligible photoactivity. Two ways of methanol activation were found. First, methoxy groups formed by reaction of methanol with coadsorbed O2 molecules or terminal OH groups are photoactive, and they turn into formaldehyde upon UV illumination. The methoxy species show an unusual C 1s core-level shift of 1.4 eV compared to methanol; their chemical assignment was verified by DFT calculations with inclusion of final-state effects. The second way of methanol activation opens at methanol coverages above 0.5 monolayer (ML), and methyl formate is produced in this reaction pathway. The adsorption of methanol in the coverage regime from 0 to 2 ML is described in detail; it is key for understanding the photocatalytic behavior at high coverages. There, a hydrogen-bonding network is established in the adsorbed methanol layer, and consequently, methanol dissociation becomes energetically more favorable. DFT calculations show that dissociation of the methanol molecule is always the key requirement for hole transfer from the substrate to the adsorbed methanol. We show that the hydrogen-bonding network established in the methanol layer dramatically changes the kinetics of proton transfer during the photoreaction.
中文翻译:

锐钛矿型TiO 2(101)上的甲醇:光催化机理的见解
通过扫描隧道显微镜(STM),程序升温脱附(TPD),X射线光电子能谱(XPS)和密度泛函理论(DFT)的组合研究了锐钛矿TiO 2(101)表面吸附的甲醇的光活性。)计算。吸附在锐钛矿(101)表面的分离的甲醇分子显示出可忽略的光活性。发现了甲醇活化的两种方法。首先,通过甲醇与共吸附的O 2反应形成的甲氧基分子或末端的OH基团具有光活性,在紫外线照射下它们会变成甲醛。与甲醇相比,甲氧基物质表现出1.4 eV的不寻常的C 1s核能级位移;通过DFT计算(包括最终状态效应)验证了它们的化学分配。甲醇活化的第二种方法是在甲醇覆盖率超过0.5单层(ML)时打开,并且在该反应路径中生成甲酸甲酯。详细描述了甲醇在0到2 ML的覆盖范围内的吸附情况。这是了解高覆盖率下光催化行为的关键。在那里,在吸附的甲醇层中建立了氢键网络,因此,甲醇的离解在能量上变得更加有利。DFT计算表明,甲醇分子的解离始终是将空穴从基质转移到吸附的甲醇的关键条件。我们表明,在甲醇层中建立的氢键网络显着改变了光反应过程中质子转移的动力学。
更新日期:2017-09-21
中文翻译:

锐钛矿型TiO 2(101)上的甲醇:光催化机理的见解
通过扫描隧道显微镜(STM),程序升温脱附(TPD),X射线光电子能谱(XPS)和密度泛函理论(DFT)的组合研究了锐钛矿TiO 2(101)表面吸附的甲醇的光活性。)计算。吸附在锐钛矿(101)表面的分离的甲醇分子显示出可忽略的光活性。发现了甲醇活化的两种方法。首先,通过甲醇与共吸附的O 2反应形成的甲氧基分子或末端的OH基团具有光活性,在紫外线照射下它们会变成甲醛。与甲醇相比,甲氧基物质表现出1.4 eV的不寻常的C 1s核能级位移;通过DFT计算(包括最终状态效应)验证了它们的化学分配。甲醇活化的第二种方法是在甲醇覆盖率超过0.5单层(ML)时打开,并且在该反应路径中生成甲酸甲酯。详细描述了甲醇在0到2 ML的覆盖范围内的吸附情况。这是了解高覆盖率下光催化行为的关键。在那里,在吸附的甲醇层中建立了氢键网络,因此,甲醇的离解在能量上变得更加有利。DFT计算表明,甲醇分子的解离始终是将空穴从基质转移到吸附的甲醇的关键条件。我们表明,在甲醇层中建立的氢键网络显着改变了光反应过程中质子转移的动力学。











































 京公网安备 11010802027423号
京公网安备 11010802027423号