当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stereoselective Synthesis of Z-Vinylsilanes via Palladium-Catalyzed Direct Intermolecular Silylation of C(sp2)–H Bonds
Organic Letters ( IF 4.9 ) Pub Date : 2017-09-21 00:00:00 , DOI: 10.1021/acs.orglett.7b02486 Jin-Long Pan 1 , Chao Chen 1 , Zhi-Gang Ma 1 , Jia Zhou 1 , Li-Ren Wang 1 , Shu-Yu Zhang 1
Organic Letters ( IF 4.9 ) Pub Date : 2017-09-21 00:00:00 , DOI: 10.1021/acs.orglett.7b02486 Jin-Long Pan 1 , Chao Chen 1 , Zhi-Gang Ma 1 , Jia Zhou 1 , Li-Ren Wang 1 , Shu-Yu Zhang 1
Affiliation
|
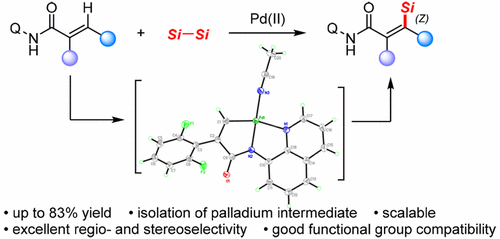
An efficient and convenient palladium-catalyzed direct intermolecular silylation of C(sp2)–H bonds by using disilanes as the silicon source with the assistance of a readily removable bidentate directing group is reported. This strategy provided a regio- and stereoselective protocol for exclusive synthesis of Z-vinylsilanes with reasonable to excellent yields and good functional group compatibility. Silylation of the isolated palladacycle intermediate revealed the Z-stereoselective pathway. Moreover, the practicality and effectiveness of this method were illustrated by a gram-scale experiment and further functionalization of the silylation product.
中文翻译:

钯催化的C(sp 2)–H键的直接分子间甲硅烷基化立体选择性合成Z-乙烯基硅烷
据报道,通过使用乙硅烷作为硅源,借助易于去除的双齿引导基团,可以有效,方便地催化钯催化的C(sp 2)-H键直接分子间甲硅烷基化。该策略为Z-乙烯基硅烷的独家合成提供了区域和立体选择性的方案,具有合理至优异的收率和良好的官能团相容性。分离的palladacycle中间体的甲硅烷基化揭示了Z-立体选择性途径。此外,该方法的实用性和有效性通过克规模的实验和甲硅烷基化产物的进一步功能化进行了说明。
更新日期:2017-09-21
中文翻译:

钯催化的C(sp 2)–H键的直接分子间甲硅烷基化立体选择性合成Z-乙烯基硅烷
据报道,通过使用乙硅烷作为硅源,借助易于去除的双齿引导基团,可以有效,方便地催化钯催化的C(sp 2)-H键直接分子间甲硅烷基化。该策略为Z-乙烯基硅烷的独家合成提供了区域和立体选择性的方案,具有合理至优异的收率和良好的官能团相容性。分离的palladacycle中间体的甲硅烷基化揭示了Z-立体选择性途径。此外,该方法的实用性和有效性通过克规模的实验和甲硅烷基化产物的进一步功能化进行了说明。











































 京公网安备 11010802027423号
京公网安备 11010802027423号