当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
First-Principles Investigation of Lithium Polysulfide Structure and Behavior in Solution
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2017-09-21 00:00:00 , DOI: 10.1021/acs.jpcc.7b04822 Ethan P. Kamphaus 1 , Perla B. Balbuena 1
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2017-09-21 00:00:00 , DOI: 10.1021/acs.jpcc.7b04822 Ethan P. Kamphaus 1 , Perla B. Balbuena 1
Affiliation
|
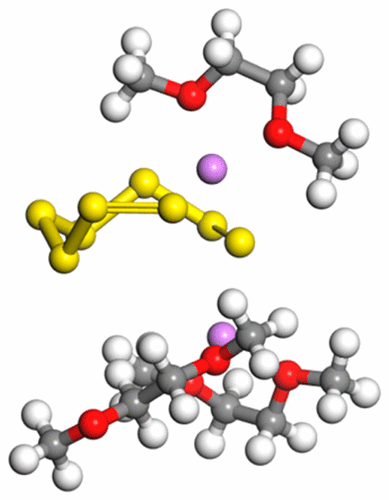
The lithium–sulfur battery is a promising next generation energy storage technology that could meet the demands of modern society with a theoretical specific energy near 2500 W h kg–1. However, this battery chemistry faces unique problems such as the parasitic polysulfide shuttle reaction, which hinders battery performance severely. This shuttle phenomenon is caused by solubilities of intermediate reaction products in the electrolyte during the reduction chemistry of the battery. With molecular simulation and computational chemistry tools, we studied the thermodynamics, solvation structure, and dynamics of the long-chain lithium polysulfide species Li2S6 and Li2S8 in dimethoxyethane and 1,3-dioxolane to gain a deeper fundamental understanding of this process. We determined the structure of the first solvation shell for Li+ as well as those of Li2S6, Li2S8 closed, and Li2S8 linear in pure solvents and solvents with extra Li+ added. The lithium polysulfide species were found not to favor dissociation and would most likely exist as fully lithiated species in solution.
中文翻译:

溶液中多硫化锂结构和行为的第一性原理研究
锂硫电池是一种很有前途的下一代储能技术,其理论比能量接近2500 W h kg –1可以满足现代社会的需求。然而,这种电池化学物质面临独特的问题,例如寄生的多硫化物往复反应,这严重地阻碍了电池的性能。这种穿梭现象是由于在电池的还原化学过程中中间反应产物在电解质中的溶解性引起的。利用分子模拟和计算化学工具,我们研究了长链多硫化锂Li 2 S 6和Li 2 S 8的热力学,溶剂化结构和动力学。在二甲氧基乙烷和1,3-二氧戊环中使用,以获得对该过程的更深层次的基础理解。我们确定的第一溶剂化层为Li的结构+以及那些的Li 2小号6,李2小号8闭合,和Li 2小号8线性在纯溶剂和溶剂与额外李+添加。发现多硫化锂物质不利于解离,最有可能以溶液中完全锂化的物质形式存在。
更新日期:2017-09-21
中文翻译:

溶液中多硫化锂结构和行为的第一性原理研究
锂硫电池是一种很有前途的下一代储能技术,其理论比能量接近2500 W h kg –1可以满足现代社会的需求。然而,这种电池化学物质面临独特的问题,例如寄生的多硫化物往复反应,这严重地阻碍了电池的性能。这种穿梭现象是由于在电池的还原化学过程中中间反应产物在电解质中的溶解性引起的。利用分子模拟和计算化学工具,我们研究了长链多硫化锂Li 2 S 6和Li 2 S 8的热力学,溶剂化结构和动力学。在二甲氧基乙烷和1,3-二氧戊环中使用,以获得对该过程的更深层次的基础理解。我们确定的第一溶剂化层为Li的结构+以及那些的Li 2小号6,李2小号8闭合,和Li 2小号8线性在纯溶剂和溶剂与额外李+添加。发现多硫化锂物质不利于解离,最有可能以溶液中完全锂化的物质形式存在。











































 京公网安备 11010802027423号
京公网安备 11010802027423号