当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Latent Porosity in Alkali-Metal M2B12F12 Salts: Structures and Rapid Room-Temperature Hydration/Dehydration Cycles
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2017-09-21 00:00:00 , DOI: 10.1021/acs.inorgchem.7b02081 Dmitry V. Peryshkov 1, 2 , Eric V. Bukovsky 1 , Matthew R. Lacroix 1 , Hui Wu 3 , Wei Zhou 3 , W. Matthew Jones 1 , Matic Lozinšek 1, 4 , Travis C. Folsom 1 , D. Luke Heyliger 1 , Terrence J. Udovic 3 , Steven H. Strauss 1
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2017-09-21 00:00:00 , DOI: 10.1021/acs.inorgchem.7b02081 Dmitry V. Peryshkov 1, 2 , Eric V. Bukovsky 1 , Matthew R. Lacroix 1 , Hui Wu 3 , Wei Zhou 3 , W. Matthew Jones 1 , Matic Lozinšek 1, 4 , Travis C. Folsom 1 , D. Luke Heyliger 1 , Terrence J. Udovic 3 , Steven H. Strauss 1
Affiliation
|
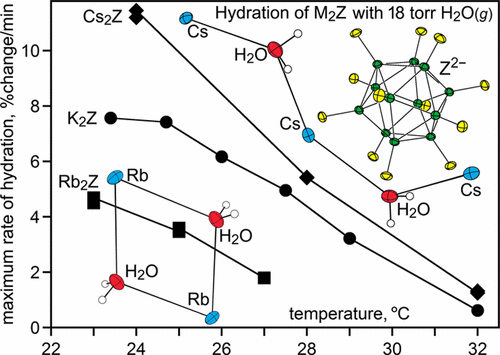
Structures of the alkali-metal hydrates Li2(H2O)4Z, LiK(H2O)4Z, Na2(H2O)3Z, and Rb2(H2O)2Z, unit cell parameters for Rb2Z and Rb2(H2O)2Z, and the density functional theory (DFT)-optimized structures of K2Z, K2(H2O)2Z, Rb2Z, Rb2(H2O)2Z, Cs2Z, and Cs2(H2O)Z are reported (Z2– = B12F122–) and compared with previously reported X-ray structures of Na2(H2O)0,4Z, K2(H2O)0,2,4Z, and Cs2(H2O)Z. Unusually rapid room-temperature hydration/dehydration cycles of several M2Z/M2(H2O)nZ salt hydrate pairs, which were studied by isothermal gravimetry, are also reported. Finely ground samples of K2Z, Rb2Z, and Cs2Z, which are not microporous, exhibited latent porosity by undergoing hydration at 24–25 °C in the presence of 18 Torr of H2O(g) to K2(H2O)2Z, Rb2(H2O)2Z, and Cs2(H2O)Z in 18, 40, and 16 min, respectively. These hydrates were dehydrated at 24–25 °C in dry N2 to the original anhydrous M2Z compounds in 61, 25, and 76 min, respectively (the exact times varied from sample to sample depending on the particle size). The hydrate Na2(H2O)2Z also exhibited latent porosity by undergoing multiple 90 min cycles of hydration to Na2(H2O)3Z and dehydration back to Na2(H2O)2Z at 23 °C. For the K2Z, Rb2Z, and Cs2Z transformations, the maximum rate of hydration (rhmax) decreased, and the absolute value of the maximum rate of dehydration (rdmax) increased, as T increased. For K2Z ↔ K2(H2O)2Z hydration/dehydration cycles with the same sample, the ratio rhmax/rdmax decreased 26 times over 8.6 °C, from 3.7 at 23.4 °C to 0.14 at 32.0 °C. For Rb2Z ↔ Rb2(H2O)2Z cycles, rhmax/rdmax decreased from 0.88 at 23 °C to 0.23 at 27 °C. For Cs2Z ↔ Cs2(H2O)Z cycles, rhmax/rdmax decreased 20 times over 8 °C, from 6.7 at 24 °C to 0.34 at 32 °C. In addition, the reversible substitution of D2O for H2O in fully hydrated Rb2(H2O)2Z in the presence of N2/16 Torr of D2O(g) was complete in only 60 min at 23 °C.
中文翻译:

碱金属M 2 B 12 F 12盐中的潜在孔隙度:结构和快速的室温水合/脱水循环
碱金属水合物Li 2(H 2 O)4 Z,LiK(H 2 O)4 Z,Na 2(H 2 O)3 Z和Rb 2(H 2 O)2 Z的结构,晶胞参数Rb 2 Z和Rb 2(H 2 O)2 Z的合成,以及K 2 Z,K 2(H 2 O)2 Z,Rb 2 Z,Rb 2(H 2)的密度泛函理论(DFT)优化结构O)2 Z,Cs报告了2 Z和Cs 2(H 2 O)Z(Z 2– = B 12 F 12 2–),并与先前报告的Na 2(H 2 O)0.4 Z,K 2的X射线结构进行了比较(H 2 O)0,2,4 Z和Cs 2(H 2 O)Z。还报道了通过等温重力法研究的几个M 2 Z / M 2(H 2 O)n Z盐水合物对的异常快速的室温水合/脱水循环。精细研磨的K 2 Z,Rb样品2 Z和Cs 2 Z(它们不是微孔的)在24托(25℃)下,在18 Torr的H 2 O(g)到K 2(H 2 O)2 Z,Rb的作用下水合,表现出潜在的孔隙度。2(H 2 O)2 Z和Cs 2(H 2 O)Z分别在18、40和16分钟内。这些水合物分别在61、25和76分钟内在干燥的N 2中于24–25°C脱水为原始的无水M 2 Z化合物(具体时间因颗粒大小而异)。水合物Na 2(H 2 O)2Z还通过在90°C下进行多个90分钟的水合生成Na 2(H 2 O)3 Z并脱水回到Na 2(H 2 O)2 Z循环,从而显示出潜在的孔隙度。对于K 2 Z,Rb 2 Z和Cs 2 Z转换,最大水合速率(rh max)减小,并且最大脱水率(rd max)的绝对值随T的增加而增加。对于相同样品的K 2 Z↔K 2(H 2 O)2 Z水合/脱水循环,比率rh max/ rd max在8.6°C时降低了26倍,从23.4°C时的3.7降至32.0°C时的0.14。对于Rb 2 Z↔Rb 2(H 2 O)2 Z循环,rh max / rd max从23°C的0.88降低到27°C的0.23。对于Cs 2 Z↔Cs 2(H 2 O)Z周期,在8°C时rh max / rd max降低20倍,从24°C时的6.7降至32°C时的0.34。此外,在存在N 2的情况下,在完全水合的Rb 2(H 2 O)2 Z中D 2 O可逆地替代H 2 O乇/ 16 d 2 O(克)在仅60分钟完成,在23℃。
更新日期:2017-09-21
中文翻译:

碱金属M 2 B 12 F 12盐中的潜在孔隙度:结构和快速的室温水合/脱水循环
碱金属水合物Li 2(H 2 O)4 Z,LiK(H 2 O)4 Z,Na 2(H 2 O)3 Z和Rb 2(H 2 O)2 Z的结构,晶胞参数Rb 2 Z和Rb 2(H 2 O)2 Z的合成,以及K 2 Z,K 2(H 2 O)2 Z,Rb 2 Z,Rb 2(H 2)的密度泛函理论(DFT)优化结构O)2 Z,Cs报告了2 Z和Cs 2(H 2 O)Z(Z 2– = B 12 F 12 2–),并与先前报告的Na 2(H 2 O)0.4 Z,K 2的X射线结构进行了比较(H 2 O)0,2,4 Z和Cs 2(H 2 O)Z。还报道了通过等温重力法研究的几个M 2 Z / M 2(H 2 O)n Z盐水合物对的异常快速的室温水合/脱水循环。精细研磨的K 2 Z,Rb样品2 Z和Cs 2 Z(它们不是微孔的)在24托(25℃)下,在18 Torr的H 2 O(g)到K 2(H 2 O)2 Z,Rb的作用下水合,表现出潜在的孔隙度。2(H 2 O)2 Z和Cs 2(H 2 O)Z分别在18、40和16分钟内。这些水合物分别在61、25和76分钟内在干燥的N 2中于24–25°C脱水为原始的无水M 2 Z化合物(具体时间因颗粒大小而异)。水合物Na 2(H 2 O)2Z还通过在90°C下进行多个90分钟的水合生成Na 2(H 2 O)3 Z并脱水回到Na 2(H 2 O)2 Z循环,从而显示出潜在的孔隙度。对于K 2 Z,Rb 2 Z和Cs 2 Z转换,最大水合速率(rh max)减小,并且最大脱水率(rd max)的绝对值随T的增加而增加。对于相同样品的K 2 Z↔K 2(H 2 O)2 Z水合/脱水循环,比率rh max/ rd max在8.6°C时降低了26倍,从23.4°C时的3.7降至32.0°C时的0.14。对于Rb 2 Z↔Rb 2(H 2 O)2 Z循环,rh max / rd max从23°C的0.88降低到27°C的0.23。对于Cs 2 Z↔Cs 2(H 2 O)Z周期,在8°C时rh max / rd max降低20倍,从24°C时的6.7降至32°C时的0.34。此外,在存在N 2的情况下,在完全水合的Rb 2(H 2 O)2 Z中D 2 O可逆地替代H 2 O乇/ 16 d 2 O(克)在仅60分钟完成,在23℃。









































 京公网安备 11010802027423号
京公网安备 11010802027423号