Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis of Salts of 1,2,5,6- and 1,4,5,8-Naphthalenetetramine
ACS Omega ( IF 4.1 ) Pub Date : 2017-09-21 00:00:00 , DOI: 10.1021/acsomega.7b01055 Sophie Langis-Barsetti 1 , Thierry Maris 1 , James D. Wuest 1
ACS Omega ( IF 4.1 ) Pub Date : 2017-09-21 00:00:00 , DOI: 10.1021/acsomega.7b01055 Sophie Langis-Barsetti 1 , Thierry Maris 1 , James D. Wuest 1
Affiliation
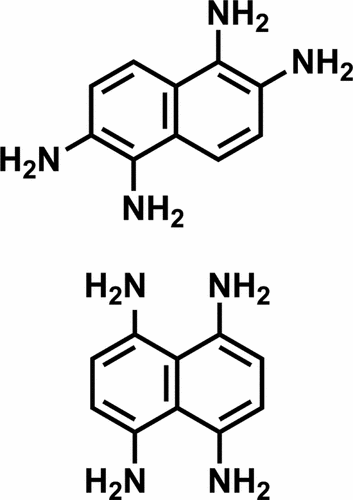
|
1,2,5,6-Naphthalenetetramine (1a), its 1,4,5,8-isomer (2a), and their salts are valuable precursors for synthesizing nitrogen-containing arenes and other targets of interest. We describe how salts of tetramines 1a and 2a can be made from simple protected derivatives of 1,5-naphthalenediamine (2d) by sequences of regioselective dinitration, deprotection, and reduction. Various shortcomings of previously reported syntheses of tetramines 1a and 2a can thereby be avoided. In addition, we report structural studies that may help clarify the mechanism of nitration and resolve an earlier controversy about the regioselectivity observed in nitrations of derivatives of 1,5-naphthalenediamine (2d).
中文翻译:

1,2,5,6-和1,4,5,8-萘四胺盐的合成
1,2,5,6-萘四胺(1a),其1,4,5,8-异构体(2a)及其盐是用于合成含氮芳烃和其他目标化合物的有价值的前体。我们描述了如何通过区域选择性二硝化,脱保护和还原的顺序,从简单的1,5-萘二胺(2d)的受保护衍生物制备四胺1a和2a的盐。先前报道的四胺1a和2a合成的各种缺点从而可以避免。此外,我们报告了结构研究,该研究可能有助于阐明硝化的机理,并解决有关在硝化1,5-萘二胺(2d)时观察到的区域选择性的较早争议。
更新日期:2017-09-21
中文翻译:

1,2,5,6-和1,4,5,8-萘四胺盐的合成
1,2,5,6-萘四胺(1a),其1,4,5,8-异构体(2a)及其盐是用于合成含氮芳烃和其他目标化合物的有价值的前体。我们描述了如何通过区域选择性二硝化,脱保护和还原的顺序,从简单的1,5-萘二胺(2d)的受保护衍生物制备四胺1a和2a的盐。先前报道的四胺1a和2a合成的各种缺点从而可以避免。此外,我们报告了结构研究,该研究可能有助于阐明硝化的机理,并解决有关在硝化1,5-萘二胺(2d)时观察到的区域选择性的较早争议。



























 京公网安备 11010802027423号
京公网安备 11010802027423号