Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Interactions on External MOF Surfaces: Desorption of Water and Ethanol from CuBDC Nanosheets
Langmuir ( IF 3.9 ) Pub Date : 2017-09-21 00:00:00 , DOI: 10.1021/acs.langmuir.7b01987 Alexander C. Elder 1 , Alexandr B. Aleksandrov 1 , Sankar Nair 1 , Thomas M. Orlando 1
Langmuir ( IF 3.9 ) Pub Date : 2017-09-21 00:00:00 , DOI: 10.1021/acs.langmuir.7b01987 Alexander C. Elder 1 , Alexandr B. Aleksandrov 1 , Sankar Nair 1 , Thomas M. Orlando 1
Affiliation
|
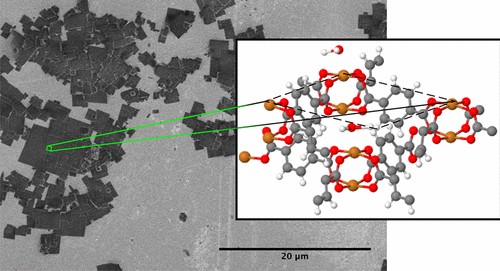
The external surfaces of metal–organic framework (MOF) materials are difficult to experimentally isolate due to the high porosities of these materials. MOF surface surrogates in the form of copper benzenedicarboxylate (CuBDC) nanosheets were synthesized using a bottom-up approach, and the surface interactions of water and ethanol were investigated by temperature-programmed desorption (TPD). A method of analysis of diffusion-influenced TPD was developed to measure the desorption properties of these porous materials. This approach also allows the extraction of diffusion coefficients from TPD data. The transmission Fourier transform infrared spectra, powder X-ray diffraction patterns, and TPD data indicate that water desorbs from CuBDC nanosheets with activation energies of 44 ± 2 kJ/mol at edge sites and 58 ± 1 kJ/mol at external surface and internal and pore sites. Ethanol desorbs with activation energies of 58 ± 1 kJ/mol at internal pore sites and 66 ± 0.4 kJ/mol at external surface sites. Co-adsorption of water and ethanol was also investigated. The presence of ethanol was found to inhibit the desorption of water, resulting in a water desorption process with an activation energy of 68 ± 0.7 kJ/mol.
中文翻译:

MOF外部表面上的相互作用:CuBDC纳米片中水和乙醇的解吸
由于金属-有机骨架(MOF)材料的高孔隙率,它们的外表面很难通过实验隔离。采用自下而上的方法合成了MOF表面替代物,形式为苯二甲酸铜(CuBDC)纳米片,并通过程序升温脱附(TPD)研究了水和乙醇的表面相互作用。开发了一种分析受扩散影响的TPD的方法,以测量这些多孔材料的解吸性能。这种方法还允许从TPD数据中提取扩散系数。透射傅立叶变换红外光谱,粉末X射线衍射图,和TPD数据表明,水从CuBDC纳米片中解吸,其活化能在边缘部位为44±2 kJ / mol,在外表面以及内部和孔部位为58±1 kJ / mol。乙醇在内部孔部位的脱附活化能为58±1 kJ / mol,在外部表面部位的脱附活化能为66±0.4 kJ / mol。还研究了水和乙醇的共吸附。发现乙醇的存在会抑制水的解吸,从而导致水的解吸过程的活化能为68±0.7 kJ / mol。
更新日期:2017-09-21
中文翻译:

MOF外部表面上的相互作用:CuBDC纳米片中水和乙醇的解吸
由于金属-有机骨架(MOF)材料的高孔隙率,它们的外表面很难通过实验隔离。采用自下而上的方法合成了MOF表面替代物,形式为苯二甲酸铜(CuBDC)纳米片,并通过程序升温脱附(TPD)研究了水和乙醇的表面相互作用。开发了一种分析受扩散影响的TPD的方法,以测量这些多孔材料的解吸性能。这种方法还允许从TPD数据中提取扩散系数。透射傅立叶变换红外光谱,粉末X射线衍射图,和TPD数据表明,水从CuBDC纳米片中解吸,其活化能在边缘部位为44±2 kJ / mol,在外表面以及内部和孔部位为58±1 kJ / mol。乙醇在内部孔部位的脱附活化能为58±1 kJ / mol,在外部表面部位的脱附活化能为66±0.4 kJ / mol。还研究了水和乙醇的共吸附。发现乙醇的存在会抑制水的解吸,从而导致水的解吸过程的活化能为68±0.7 kJ / mol。



























 京公网安备 11010802027423号
京公网安备 11010802027423号