当前位置:
X-MOL 学术
›
Eur. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
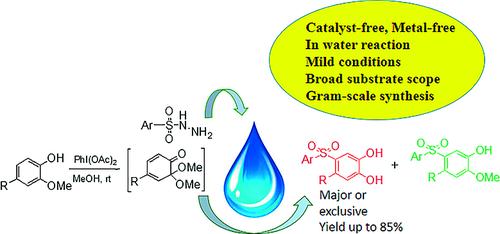
Catalyst-Free Sulfonylation of 2-Methoxyphenols: Facile One-Pot Synthesis of (Arylsulfonyl)catechols in Aqueous Media
European Journal of Organic Chemistry ( IF 2.5 ) Pub Date : 2017-09-21 , DOI: 10.1002/ejoc.201701038 Neha Taneja 1 , Rama Krishna Peddinti 1
European Journal of Organic Chemistry ( IF 2.5 ) Pub Date : 2017-09-21 , DOI: 10.1002/ejoc.201701038 Neha Taneja 1 , Rama Krishna Peddinti 1
Affiliation
|
A new water-assisted carbon–sulfur bond-formation strategy is described for direct access to highly valuable (arylsulfonyl)catechols. This scalable transformation is remarkable, as a tandem dearomatization/sulfonylation and hydroxylation process enabled the title compounds to be formed in one pot under catalyst-free conditions. Mechanistic studies revealed that water plays a key role in the synthesis of the catechols. The sulfonylation operates under very mild conditions, shows a broad substrate scope, gives high conversions, and can be carried out on a gram scale. Thus, this method represents a green, efficient method for carbon–sulfur bond formation. The aryl sulfones were also further transformed into molecules that are important for drug discovery.
中文翻译:

2-甲氧基苯酚的无催化剂磺酰化:(芳基磺酰基)儿茶酚在水性介质中的简便一锅法合成
描述了一种新的水辅助碳硫键形成策略,用于直接获得高价值的(芳基磺酰基)儿茶酚。这种可扩展的转化是显着的,因为串联脱芳构化/磺酰化和羟基化过程使标题化合物能够在无催化剂条件下在一个锅中形成。机理研究表明,水在儿茶酚的合成中起着关键作用。磺酰化反应在非常温和的条件下进行,底物范围广,转化率高,并且可以在克规模上进行。因此,该方法代表了一种绿色、有效的碳硫键形成方法。芳基砜也被进一步转化为对药物发现很重要的分子。
更新日期:2017-09-21
中文翻译:

2-甲氧基苯酚的无催化剂磺酰化:(芳基磺酰基)儿茶酚在水性介质中的简便一锅法合成
描述了一种新的水辅助碳硫键形成策略,用于直接获得高价值的(芳基磺酰基)儿茶酚。这种可扩展的转化是显着的,因为串联脱芳构化/磺酰化和羟基化过程使标题化合物能够在无催化剂条件下在一个锅中形成。机理研究表明,水在儿茶酚的合成中起着关键作用。磺酰化反应在非常温和的条件下进行,底物范围广,转化率高,并且可以在克规模上进行。因此,该方法代表了一种绿色、有效的碳硫键形成方法。芳基砜也被进一步转化为对药物发现很重要的分子。










































 京公网安备 11010802027423号
京公网安备 11010802027423号