当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
AgOTf-catalyzed sequential synthesis of 4-isoquinolones via oxidative ring opening of aziridines and aza-Michael addition
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2017-09-12 00:00:00 , DOI: 10.1039/c7ob02167d Siyang Xing 1, 2, 3, 4, 5 , Hong Cui 1, 2, 3, 4, 5 , Nan Gu 1, 2, 3, 4, 5 , Ying Li 1, 2, 3, 4, 5 , Kui Wang 1, 2, 3, 4, 5 , Dawei Tian 1, 2, 3, 4, 5 , Jiajing Qin 1, 2, 3, 4, 5 , Qiaoyang Liu 1, 2, 3, 4, 5
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2017-09-12 00:00:00 , DOI: 10.1039/c7ob02167d Siyang Xing 1, 2, 3, 4, 5 , Hong Cui 1, 2, 3, 4, 5 , Nan Gu 1, 2, 3, 4, 5 , Ying Li 1, 2, 3, 4, 5 , Kui Wang 1, 2, 3, 4, 5 , Dawei Tian 1, 2, 3, 4, 5 , Jiajing Qin 1, 2, 3, 4, 5 , Qiaoyang Liu 1, 2, 3, 4, 5
Affiliation
|
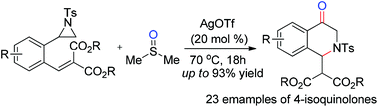
An efficient AgOTf-catalyzed sequential reaction involving the oxidative ring-opening of aziridines by DMSO and aza-Michael addition has been developed. A series of 2,3-dihydro-4(1H)-isoquinolones were afforded in moderate to good yields by the formation of one new C![[double bond, length as m-dash]](http://www.rsc.org/images/entities/char_e001.gif) O bond and one new C–N bond. The features of this sequential reaction include high bonding efficiency, use of a catalytic amount of catalysts, a broad substrate scope and mild conditions. This methodology provides a good choice for constructing the libraries of 2,3-dihydro-4(1H)-isoquinolones.
O bond and one new C–N bond. The features of this sequential reaction include high bonding efficiency, use of a catalytic amount of catalysts, a broad substrate scope and mild conditions. This methodology provides a good choice for constructing the libraries of 2,3-dihydro-4(1H)-isoquinolones.
中文翻译:

AgOTf通过氮丙啶的氧化开环和氮杂-迈克尔加成反应催化4-异喹啉酮的顺序合成
已经开发了一种有效的AgOTf催化的顺序反应,该反应涉及通过DMSO和氮杂-迈克尔加成反应使氮丙啶类化合物的氧化开环。通过形成一个新的C O键和一个新的C–N键,可以中等到良好的产率获得一系列的2,3-二氢-4(1 H)-异喹诺酮类药物![[双键,长度为m-破折号]](http://www.rsc.org/images/entities/char_e001.gif) 。该顺序反应的特征包括高键合效率,使用催化量的催化剂,广泛的底物范围和温和的条件。此方法为构建2,3-二氢-4(1 H)-异喹诺酮文库提供了一个不错的选择。
。该顺序反应的特征包括高键合效率,使用催化量的催化剂,广泛的底物范围和温和的条件。此方法为构建2,3-二氢-4(1 H)-异喹诺酮文库提供了一个不错的选择。
更新日期:2017-09-21
![[double bond, length as m-dash]](http://www.rsc.org/images/entities/char_e001.gif) O bond and one new C–N bond. The features of this sequential reaction include high bonding efficiency, use of a catalytic amount of catalysts, a broad substrate scope and mild conditions. This methodology provides a good choice for constructing the libraries of 2,3-dihydro-4(1H)-isoquinolones.
O bond and one new C–N bond. The features of this sequential reaction include high bonding efficiency, use of a catalytic amount of catalysts, a broad substrate scope and mild conditions. This methodology provides a good choice for constructing the libraries of 2,3-dihydro-4(1H)-isoquinolones.
中文翻译:

AgOTf通过氮丙啶的氧化开环和氮杂-迈克尔加成反应催化4-异喹啉酮的顺序合成
已经开发了一种有效的AgOTf催化的顺序反应,该反应涉及通过DMSO和氮杂-迈克尔加成反应使氮丙啶类化合物的氧化开环。通过形成一个新的C O键和一个新的C–N键,可以中等到良好的产率获得一系列的2,3-二氢-4(1 H)-异喹诺酮类药物
![[双键,长度为m-破折号]](http://www.rsc.org/images/entities/char_e001.gif) 。该顺序反应的特征包括高键合效率,使用催化量的催化剂,广泛的底物范围和温和的条件。此方法为构建2,3-二氢-4(1 H)-异喹诺酮文库提供了一个不错的选择。
。该顺序反应的特征包括高键合效率,使用催化量的催化剂,广泛的底物范围和温和的条件。此方法为构建2,3-二氢-4(1 H)-异喹诺酮文库提供了一个不错的选择。











































 京公网安备 11010802027423号
京公网安备 11010802027423号