当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanism of C–C and C–H bond cleavage in ethanol oxidation reaction on Cu2O(111): a DFT-D and DFT+U study
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2017-09-04 00:00:00 , DOI: 10.1039/c7cp04630h Han Xu 1, 2, 3, 4, 5 , Bei Miao 1, 2, 3, 4, 5 , Minhua Zhang 1, 2, 3, 4, 5 , Yifei Chen 1, 2, 3, 4, 5 , Lichang Wang 1, 2, 3, 4, 5
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2017-09-04 00:00:00 , DOI: 10.1039/c7cp04630h Han Xu 1, 2, 3, 4, 5 , Bei Miao 1, 2, 3, 4, 5 , Minhua Zhang 1, 2, 3, 4, 5 , Yifei Chen 1, 2, 3, 4, 5 , Lichang Wang 1, 2, 3, 4, 5
Affiliation
|
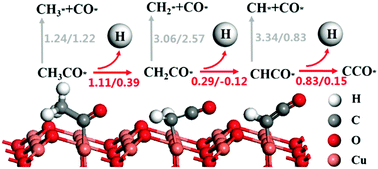
The performance of transition metal catalysts for ethanol oxidation reaction (EOR) in direct ethanol fuel cells (DEFCs) may be greatly affected by their oxidation. However, the specific effect and catalytic mechanism for EOR of transition metal oxides are still unclear and deserve in-depth exploitation. Copper as a potential anode catalyst can be easily oxidized in air. Thus, in this study, we investigated C–C and C–H bond cleavage reactions of CHxCO (x = 1, 2, 3) species in EOR on Cu2O(111) using PBE+U calculations, as well as the specific effect of +U correction on the process of adsorption and reaction on Cu2O(111). It was revealed that the catalytic performance of Cu2O(111) for EOR was restrained compared with that of Cu(100). Except for the C–H cleavage of CH2CO, all the reaction barriers for C–C and C–H cleavage were higher than those on Cu(100). The most probable pathway for CH3CO to CHCO on Cu2O(111) was the continuous dehydrogenation reaction. Besides, the barrier for C–C bond cleavage increased due to the loss of H atoms in the intermediate. Moreover, by the comparison of the traditional GGA/PBE method and the PBE+U method, it could be concluded that C–C cleavage barriers would be underestimated without +U correction, while C–H cleavage barriers would be overestimated. +U correction was proved to be necessary, and the reaction barriers and the values of the Hubbard U parameter had a proper linear relationship.
中文翻译:

Cu 2 O(111)上乙醇氧化反应中C–C和C–H键断裂的机理:DFT-D和DFT + U研究
直接乙醇燃料电池(DEFC)中用于乙醇氧化反应(EOR)的过渡金属催化剂的性能可能会受到其氧化的很大影响。然而,目前尚不清楚过渡金属氧化物EOR的具体作用和催化机理,值得深入研究。铜作为潜在的阳极催化剂很容易在空气中被氧化。因此,在该研究中,我们调查CH的C-C和C-H键断裂反应X CO(X = 1,2,3)在EOR物种对Cu 2使用PBE + O(111)Ü计算,以及所述+ U校正对Cu 2 O(111)吸附和反应过程的特定影响。揭示了铜的催化性能与Cu(100)相比,EOR的2 O(111)受到抑制。除了CH 2 CO的CH裂解以外,CC和CH裂解的所有反应障碍均高于Cu(100)。在Cu 2 O(111)上CH 3 CO转化为CHCO的最可能途径是连续脱氢反应。此外,由于中间体中H原子的损失,CC键断裂的障碍也增加了。此外,通过将传统的GGA / PBE方法与PBE + U方法进行比较,可以得出结论,如果不进行+ U校正,将低估C–C裂解障碍,而C–H裂解障碍则会被高估。+ U事实证明校正是必要的,并且反应障碍和Hubbard U参数的值具有适当的线性关系。
更新日期:2017-09-21
中文翻译:

Cu 2 O(111)上乙醇氧化反应中C–C和C–H键断裂的机理:DFT-D和DFT + U研究
直接乙醇燃料电池(DEFC)中用于乙醇氧化反应(EOR)的过渡金属催化剂的性能可能会受到其氧化的很大影响。然而,目前尚不清楚过渡金属氧化物EOR的具体作用和催化机理,值得深入研究。铜作为潜在的阳极催化剂很容易在空气中被氧化。因此,在该研究中,我们调查CH的C-C和C-H键断裂反应X CO(X = 1,2,3)在EOR物种对Cu 2使用PBE + O(111)Ü计算,以及所述+ U校正对Cu 2 O(111)吸附和反应过程的特定影响。揭示了铜的催化性能与Cu(100)相比,EOR的2 O(111)受到抑制。除了CH 2 CO的CH裂解以外,CC和CH裂解的所有反应障碍均高于Cu(100)。在Cu 2 O(111)上CH 3 CO转化为CHCO的最可能途径是连续脱氢反应。此外,由于中间体中H原子的损失,CC键断裂的障碍也增加了。此外,通过将传统的GGA / PBE方法与PBE + U方法进行比较,可以得出结论,如果不进行+ U校正,将低估C–C裂解障碍,而C–H裂解障碍则会被高估。+ U事实证明校正是必要的,并且反应障碍和Hubbard U参数的值具有适当的线性关系。











































 京公网安备 11010802027423号
京公网安备 11010802027423号