当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Dynamic protein self-assembly driven by host–guest chemistry and the folding–unfolding feature of a mutually exclusive protein
Chemical Communications ( IF 4.3 ) Pub Date : 2017-09-04 00:00:00 , DOI: 10.1039/c7cc05745h Ruidi Wang 1, 2, 3, 4, 5 , Shanpeng Qiao 1, 2, 3, 4, 5 , Linlu Zhao 1, 2, 3, 4, 5 , Chunxi Hou 1, 2, 3, 4, 5 , Xiumei Li 1, 2, 3, 4, 5 , Yao Liu 1, 2, 3, 4, 5 , Quan Luo 1, 2, 3, 4, 5 , Jiayun Xu 1, 2, 3, 4, 5 , Hongbin Li 6, 7, 8, 9 , Junqiu Liu 1, 2, 3, 4, 5
Chemical Communications ( IF 4.3 ) Pub Date : 2017-09-04 00:00:00 , DOI: 10.1039/c7cc05745h Ruidi Wang 1, 2, 3, 4, 5 , Shanpeng Qiao 1, 2, 3, 4, 5 , Linlu Zhao 1, 2, 3, 4, 5 , Chunxi Hou 1, 2, 3, 4, 5 , Xiumei Li 1, 2, 3, 4, 5 , Yao Liu 1, 2, 3, 4, 5 , Quan Luo 1, 2, 3, 4, 5 , Jiayun Xu 1, 2, 3, 4, 5 , Hongbin Li 6, 7, 8, 9 , Junqiu Liu 1, 2, 3, 4, 5
Affiliation
|
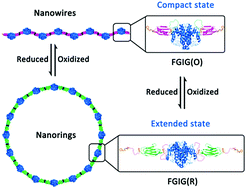
A novel exploration utilizing a well-designed fusion protein containing a redox stimuli-responsive domain was developed to construct dynamic protein self-assemblies induced by cucurbit[8]uril-based supramolecular interactions. The reversible interconversion of the morphology of the assemblies between nanowires and nanorings was regulated precisely by redox conditions.
中文翻译:

宿主-客体化学和相互排斥的蛋白质的折叠-展开特征驱动动态蛋白质自组装
一种新颖的探索,利用精心设计的包含氧化还原刺激响应域的融合蛋白,构建了基于葫芦丝[8] uril的超分子相互作用诱导的动态蛋白自组装体。纳米线和纳米环之间组件形态的可逆相互转换受氧化还原条件的精确调节。
更新日期:2017-09-21
中文翻译:

宿主-客体化学和相互排斥的蛋白质的折叠-展开特征驱动动态蛋白质自组装
一种新颖的探索,利用精心设计的包含氧化还原刺激响应域的融合蛋白,构建了基于葫芦丝[8] uril的超分子相互作用诱导的动态蛋白自组装体。纳米线和纳米环之间组件形态的可逆相互转换受氧化还原条件的精确调节。











































 京公网安备 11010802027423号
京公网安备 11010802027423号