当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nb2©Au6: a molecular wheel with a short NbNb triple bond coordinated by an Au6 ring and reinforced by σ aromaticity
Chemical Science ( IF 7.6 ) Pub Date : 2017-09-15 00:00:00 , DOI: 10.1039/c7sc02881d Tian Jian 1, 2, 3, 4 , Ling Fung Cheung 1, 2, 3, 4 , Joseph Czekner 1, 2, 3, 4 , Teng-Teng Chen 1, 2, 3, 4 , Gary V. Lopez 1, 2, 3, 4 , Wei-Li Li 1, 2, 3, 4 , Lai-Sheng Wang 1, 2, 3, 4
Chemical Science ( IF 7.6 ) Pub Date : 2017-09-15 00:00:00 , DOI: 10.1039/c7sc02881d Tian Jian 1, 2, 3, 4 , Ling Fung Cheung 1, 2, 3, 4 , Joseph Czekner 1, 2, 3, 4 , Teng-Teng Chen 1, 2, 3, 4 , Gary V. Lopez 1, 2, 3, 4 , Wei-Li Li 1, 2, 3, 4 , Lai-Sheng Wang 1, 2, 3, 4
Affiliation
|
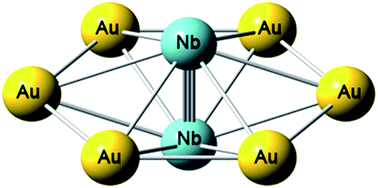
We report a photoelectron spectroscopy and high-resolution photoelectron imaging study of a bimetallic Nb2Au6− cluster. Theoretical calculations, in conjunction with the experimental data, reveal that Nb2Au6−/0 possess high-symmetry D6h structures featuring a Nb–Nb axis coordinated equatorially by an Au6 ring. Chemical bonding analyses show that there are two π bonds and one σ bond in the Nb2 moiety in Nb2©Au6, as well as five totally delocalized σ bonds. The Nb![[triple bond, length as m-dash]](http://www.rsc.org/images/entities/char_e002.gif) Nb triple bond is strengthened significantly by the delocalized σ bonds, resulting in an extremely short Nb–Nb bond length comparable to the quintuple bond in gaseous Nb2. The totally delocalized σ bonding in Nb2©Au6 is reminiscent of σ aromaticity, representing a new bonding mode in metal–ligand systems. The unusually short Nb–Nb bond length in Nb2©Au6 shows that the Au6 ring can serve as a bridging ligand to facilitate multiple bonding in transition metal dimers via delocalized σ bonding.
Nb triple bond is strengthened significantly by the delocalized σ bonds, resulting in an extremely short Nb–Nb bond length comparable to the quintuple bond in gaseous Nb2. The totally delocalized σ bonding in Nb2©Au6 is reminiscent of σ aromaticity, representing a new bonding mode in metal–ligand systems. The unusually short Nb–Nb bond length in Nb2©Au6 shows that the Au6 ring can serve as a bridging ligand to facilitate multiple bonding in transition metal dimers via delocalized σ bonding.
中文翻译:

Nb 2 ©Au 6:具有短Nb![[三重键,长度为m-破折号]](http://www.rsc.org/images/entities/h2_char_e002.gif) Nb三键的分子轮,该分子轮由Au 6环配位并由σ芳香性增强
Nb三键的分子轮,该分子轮由Au 6环配位并由σ芳香性增强
我们报告的双金属铌的光电子能谱和高分辨率光电子成像研究2金6 -集群。理论计算和实验数据表明,Nb 2 Au 6- / 0具有高对称性的D 6h结构,其Nb–Nb轴由Au 6环赤道配位。化学键分析显示,Nb 2 ©Au 6的Nb 2部分中有两个π键和一个σ键,以及五个完全离域的σ键。铌![[三重键,长度为m-破折号]](http://www.rsc.org/images/entities/char_e002.gif) Nb三键通过离域σ键显着增强,导致Nb–Nb键长度极短,可与气态Nb 2中的五重键相媲美。Nb 2 ©Au 6中的完全离域的σ键使人联想到σ芳香性,代表了金属-配体系统中的一种新的键合模式。Nb 2 ©Au 6中异常短的Nb–Nb键长度表明,Au 6环可作为桥联配体,通过离域σ键促进过渡金属二聚体中的多重键合。
Nb三键通过离域σ键显着增强,导致Nb–Nb键长度极短,可与气态Nb 2中的五重键相媲美。Nb 2 ©Au 6中的完全离域的σ键使人联想到σ芳香性,代表了金属-配体系统中的一种新的键合模式。Nb 2 ©Au 6中异常短的Nb–Nb键长度表明,Au 6环可作为桥联配体,通过离域σ键促进过渡金属二聚体中的多重键合。
更新日期:2017-09-21
![[triple bond, length as m-dash]](http://www.rsc.org/images/entities/char_e002.gif) Nb triple bond is strengthened significantly by the delocalized σ bonds, resulting in an extremely short Nb–Nb bond length comparable to the quintuple bond in gaseous Nb2. The totally delocalized σ bonding in Nb2©Au6 is reminiscent of σ aromaticity, representing a new bonding mode in metal–ligand systems. The unusually short Nb–Nb bond length in Nb2©Au6 shows that the Au6 ring can serve as a bridging ligand to facilitate multiple bonding in transition metal dimers via delocalized σ bonding.
Nb triple bond is strengthened significantly by the delocalized σ bonds, resulting in an extremely short Nb–Nb bond length comparable to the quintuple bond in gaseous Nb2. The totally delocalized σ bonding in Nb2©Au6 is reminiscent of σ aromaticity, representing a new bonding mode in metal–ligand systems. The unusually short Nb–Nb bond length in Nb2©Au6 shows that the Au6 ring can serve as a bridging ligand to facilitate multiple bonding in transition metal dimers via delocalized σ bonding.
中文翻译:

Nb 2 ©Au 6:具有短Nb
![[三重键,长度为m-破折号]](http://www.rsc.org/images/entities/h2_char_e002.gif) Nb三键的分子轮,该分子轮由Au 6环配位并由σ芳香性增强
Nb三键的分子轮,该分子轮由Au 6环配位并由σ芳香性增强
我们报告的双金属铌的光电子能谱和高分辨率光电子成像研究2金6 -集群。理论计算和实验数据表明,Nb 2 Au 6- / 0具有高对称性的D 6h结构,其Nb–Nb轴由Au 6环赤道配位。化学键分析显示,Nb 2 ©Au 6的Nb 2部分中有两个π键和一个σ键,以及五个完全离域的σ键。铌
![[三重键,长度为m-破折号]](http://www.rsc.org/images/entities/char_e002.gif) Nb三键通过离域σ键显着增强,导致Nb–Nb键长度极短,可与气态Nb 2中的五重键相媲美。Nb 2 ©Au 6中的完全离域的σ键使人联想到σ芳香性,代表了金属-配体系统中的一种新的键合模式。Nb 2 ©Au 6中异常短的Nb–Nb键长度表明,Au 6环可作为桥联配体,通过离域σ键促进过渡金属二聚体中的多重键合。
Nb三键通过离域σ键显着增强,导致Nb–Nb键长度极短,可与气态Nb 2中的五重键相媲美。Nb 2 ©Au 6中的完全离域的σ键使人联想到σ芳香性,代表了金属-配体系统中的一种新的键合模式。Nb 2 ©Au 6中异常短的Nb–Nb键长度表明,Au 6环可作为桥联配体,通过离域σ键促进过渡金属二聚体中的多重键合。











































 京公网安备 11010802027423号
京公网安备 11010802027423号