当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Biosynthesis of methyl-proline containing griselimycins, natural products with anti-tuberculosis activity
Chemical Science ( IF 7.6 ) Pub Date : 2017-08-03 00:00:00 , DOI: 10.1039/c7sc02622f Peer Lukat 1, 2, 3, 4, 5 , Yohei Katsuyama 1, 2, 3, 4, 5 , Silke Wenzel 1, 2, 3, 4, 5 , Tina Binz 1, 2, 3, 4, 5 , Claudia König 5, 6, 7, 8 , Wulf Blankenfeldt 5, 9, 10, 11, 12 , Mark Brönstrup 5, 10, 11, 13 , Rolf Müller 1, 2, 3, 4, 5
Chemical Science ( IF 7.6 ) Pub Date : 2017-08-03 00:00:00 , DOI: 10.1039/c7sc02622f Peer Lukat 1, 2, 3, 4, 5 , Yohei Katsuyama 1, 2, 3, 4, 5 , Silke Wenzel 1, 2, 3, 4, 5 , Tina Binz 1, 2, 3, 4, 5 , Claudia König 5, 6, 7, 8 , Wulf Blankenfeldt 5, 9, 10, 11, 12 , Mark Brönstrup 5, 10, 11, 13 , Rolf Müller 1, 2, 3, 4, 5
Affiliation
|
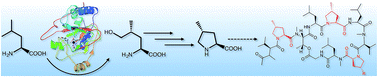
Griselimycins (GMs) are depsidecapeptides with superb anti-tuberculosis activity. They contain up to three (2S,4R)-4-methyl-prolines (4-MePro), of which one blocks oxidative degradation and increases metabolic stability in animal models. The natural congener with this substitution is only a minor component in fermentation cultures. We showed that this product can be significantly increased by feeding the reaction with 4-MePro and we investigated the molecular basis of 4-MePro biosynthesis and incorporation. We identified the GM biosynthetic gene cluster as encoding a nonribosomal peptide synthetase and a sub-operon for 4-MePro formation. Using heterologous expression, gene inactivation, and in vitro experiments, we showed that 4-MePro is generated by leucine hydroxylation, oxidation to an aldehyde, and ring closure with subsequent reduction. The crystal structures of the leucine hydroxylase GriE have been determined in complex with substrates and products, providing insight into the stereospecificity of the reaction.
中文翻译:

含甲基脯氨酸的格林霉素(具有抗结核活性的天然产物)的生物合成
格林霉素(GMs)是具有极好的抗结核活性的depsidecapepteptides。它们包含多达三个(2 S,4 R)-4-甲基脯氨酸(4-MePro),其中一个在动物模型中可以阻止氧化降解并提高代谢稳定性。具有这种取代的天然同源物仅是发酵培养物中的次要成分。我们表明,通过向反应物中加入4-MePro可以显着增加该产物,并且我们研究了4-MePro生物合成和掺入的分子基础。我们确定了GM生物合成基因簇编码为4-MePro形成的非核糖体肽合成酶和一个亚操纵子。使用异源表达,基因失活和体外实验中,我们证明了4-MePro是由亮氨酸羟基化,氧化为醛并随后进行环还原而生成的。已确定亮氨酸羟化酶GriE的晶体结构与底物和产物复合,从而提供了对反应立体特异性的认识。
更新日期:2017-09-21
中文翻译:

含甲基脯氨酸的格林霉素(具有抗结核活性的天然产物)的生物合成
格林霉素(GMs)是具有极好的抗结核活性的depsidecapepteptides。它们包含多达三个(2 S,4 R)-4-甲基脯氨酸(4-MePro),其中一个在动物模型中可以阻止氧化降解并提高代谢稳定性。具有这种取代的天然同源物仅是发酵培养物中的次要成分。我们表明,通过向反应物中加入4-MePro可以显着增加该产物,并且我们研究了4-MePro生物合成和掺入的分子基础。我们确定了GM生物合成基因簇编码为4-MePro形成的非核糖体肽合成酶和一个亚操纵子。使用异源表达,基因失活和体外实验中,我们证明了4-MePro是由亮氨酸羟基化,氧化为醛并随后进行环还原而生成的。已确定亮氨酸羟化酶GriE的晶体结构与底物和产物复合,从而提供了对反应立体特异性的认识。











































 京公网安备 11010802027423号
京公网安备 11010802027423号