Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structure of FUS Protein Fibrils and Its Relevance to Self-Assembly and Phase Separation of Low-Complexity Domains.
Cell ( IF 45.5 ) Pub Date : 2017-Oct-19 , DOI: 10.1016/j.cell.2017.08.048 Dylan T Murray 1 , Masato Kato 2 , Yi Lin 2 , Kent R Thurber 3 , Ivan Hung 4 , Steven L McKnight 2 , Robert Tycko 3
Cell ( IF 45.5 ) Pub Date : 2017-Oct-19 , DOI: 10.1016/j.cell.2017.08.048 Dylan T Murray 1 , Masato Kato 2 , Yi Lin 2 , Kent R Thurber 3 , Ivan Hung 4 , Steven L McKnight 2 , Robert Tycko 3
Affiliation
|
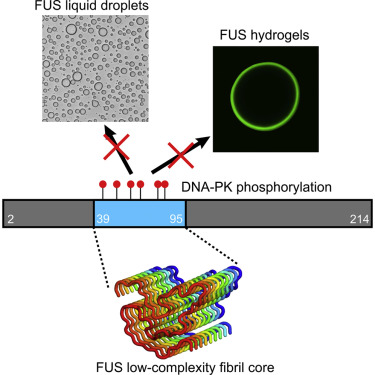
Polymerization and phase separation of proteins containing low-complexity (LC) domains are important factors in gene expression, mRNA processing and trafficking, and localization of translation. We have used solid-state nuclear magnetic resonance methods to characterize the molecular structure of self-assembling fibrils formed by the LC domain of the fused in sarcoma (FUS) RNA-binding protein. From the 214-residue LC domain of FUS (FUS-LC), a segment of only 57 residues forms the fibril core, while other segments remain dynamically disordered. Unlike pathogenic amyloid fibrils, FUS-LC fibrils lack hydrophobic interactions within the core and are not polymorphic at the molecular structural level. Phosphorylation of core-forming residues by DNA-dependent protein kinase blocks binding of soluble FUS-LC to FUS-LC hydrogels and dissolves phase-separated, liquid-like FUS-LC droplets. These studies offer a structural basis for understanding LC domain self-assembly, phase separation, and regulation by post-translational modification.
中文翻译:

FUS 蛋白原纤维的结构及其与低复杂性结构域自组装和相分离的相关性。
含有低复杂性 (LC) 结构域的蛋白质的聚合和相分离是基因表达、mRNA 加工和运输以及翻译定位的重要因素。我们使用固态核磁共振方法来表征由肉瘤融合(FUS)RNA结合蛋白的LC结构域形成的自组装原纤维的分子结构。从 FUS (FUS-LC) 的 214 个残基 LC 结构域中,只有 57 个残基的片段形成原纤维核心,而其他片段则保持动态无序。与致病性淀粉样原纤维不同,FUS-LC原纤维在核心内缺乏疏水相互作用,并且在分子结构水平上不具有多态性。DNA 依赖性蛋白激酶对核心形成残基的磷酸化会阻止可溶性 FUS-LC 与 FUS-LC 水凝胶的结合,并溶解相分离的液体状 FUS-LC 液滴。这些研究为理解 LC 结构域自组装、相分离和翻译后修饰调节提供了结构基础。
更新日期:2017-09-21
中文翻译:

FUS 蛋白原纤维的结构及其与低复杂性结构域自组装和相分离的相关性。
含有低复杂性 (LC) 结构域的蛋白质的聚合和相分离是基因表达、mRNA 加工和运输以及翻译定位的重要因素。我们使用固态核磁共振方法来表征由肉瘤融合(FUS)RNA结合蛋白的LC结构域形成的自组装原纤维的分子结构。从 FUS (FUS-LC) 的 214 个残基 LC 结构域中,只有 57 个残基的片段形成原纤维核心,而其他片段则保持动态无序。与致病性淀粉样原纤维不同,FUS-LC原纤维在核心内缺乏疏水相互作用,并且在分子结构水平上不具有多态性。DNA 依赖性蛋白激酶对核心形成残基的磷酸化会阻止可溶性 FUS-LC 与 FUS-LC 水凝胶的结合,并溶解相分离的液体状 FUS-LC 液滴。这些研究为理解 LC 结构域自组装、相分离和翻译后修饰调节提供了结构基础。











































 京公网安备 11010802027423号
京公网安备 11010802027423号