当前位置:
X-MOL 学术
›
Anal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Dynamics of Silver Nanoparticles in Aqueous Solution in the Presence of Metal Ions
Analytical Chemistry ( IF 6.7 ) Pub Date : 2017-09-21 00:00:00 , DOI: 10.1021/acs.analchem.7b01470 Kamonwad Ngamchuea 1 , Christopher Batchelor-McAuley 1 , Stanislav V. Sokolov 1 , Richard G. Compton 1
Analytical Chemistry ( IF 6.7 ) Pub Date : 2017-09-21 00:00:00 , DOI: 10.1021/acs.analchem.7b01470 Kamonwad Ngamchuea 1 , Christopher Batchelor-McAuley 1 , Stanislav V. Sokolov 1 , Richard G. Compton 1
Affiliation
|
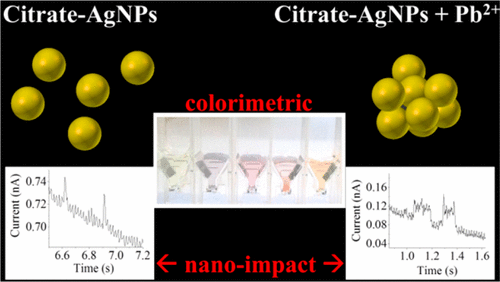
Using a combined UV–vis, DLS, and electrochemical approach, this work experimentally studies the physical origin of the observed colorimetric sensitivity of aqueous silver nanoparticles toward divalent metal ions. In the presence of Pb2+, AgNPs are slow to reversibly form agglomerates (the time scale of the reverse deagglomeration process is of the order of hours). This agglomeration is shown to be induced by complex formation between Pb2+ and citrate groups localized on the AgNPs, reducing surface charges (zeta-potential) and hence electrostatic repulsion between the AgNPs. Other divalent metal ions including Ca2+, Cd2+, Zn2+, Ni2+, Co2+, and Sn2+ are also studied, and the resulting sizes of the AgNPs clusters and the extents of the UV−vis spectrum red-shift in λmax have a strong positive correlation with the metal–ligand (citrate) complex formation constant (Kf). This work thus serves as a guide for the selection of capping agents on the basis of Kf and demonstrates the correlation between sizes and spectrophotometric as well as electrochemical responses of the AgNPs clusters. Importantly, we give further physical insights into the size-dependent properties of AgNPs and emphasize the difference between theoretical and experimental values of extinction coefficients, where the latter is affected by the angle-dependent scattering intensities and the measurement technique used.
中文翻译:

金属离子存在下水溶液中银纳米粒子的动力学
通过结合使用紫外可见光,DLS和电化学方法,这项工作通过实验研究了所观察到的水性银纳米颗粒对二价金属离子的比色敏感性的物理来源。在Pb 2+的存在下,AgNP缓慢而可逆地形成团聚体(逆解聚团过程的时间尺度约为几小时)。已表明,这种聚集是由于Pb 2+与位于AgNPs上的柠檬酸盐基团之间的络合物形成而引起的,从而降低了表面电荷(ζ电位),从而降低了AgNPs之间的静电排斥力。其他二价金属离子,包括Ca 2 +,Cd 2 +,Zn 2 +,Ni 2 +,Co 2+和Sn2+也进行了研究,并将所得的的AgNPs簇的大小和UV-Vis光谱的程度红移在λ最大具有与金属配体(柠檬酸盐)复合物形成常数(一个很强的正相关ķ ˚F)。因此,这项工作可作为基于K f选择封端剂的指南。并证明了AgNPs团簇的尺寸与分光光度以及电化学响应之间的相关性。重要的是,我们对AgNPs的尺寸依赖性特性提供了进一步的物理见解,并强调了消光系数的理论值与实验值之间的差异,其中消光系数受角度相关的散射强度和所使用的测量技术的影响。
更新日期:2017-09-21
中文翻译:

金属离子存在下水溶液中银纳米粒子的动力学
通过结合使用紫外可见光,DLS和电化学方法,这项工作通过实验研究了所观察到的水性银纳米颗粒对二价金属离子的比色敏感性的物理来源。在Pb 2+的存在下,AgNP缓慢而可逆地形成团聚体(逆解聚团过程的时间尺度约为几小时)。已表明,这种聚集是由于Pb 2+与位于AgNPs上的柠檬酸盐基团之间的络合物形成而引起的,从而降低了表面电荷(ζ电位),从而降低了AgNPs之间的静电排斥力。其他二价金属离子,包括Ca 2 +,Cd 2 +,Zn 2 +,Ni 2 +,Co 2+和Sn2+也进行了研究,并将所得的的AgNPs簇的大小和UV-Vis光谱的程度红移在λ最大具有与金属配体(柠檬酸盐)复合物形成常数(一个很强的正相关ķ ˚F)。因此,这项工作可作为基于K f选择封端剂的指南。并证明了AgNPs团簇的尺寸与分光光度以及电化学响应之间的相关性。重要的是,我们对AgNPs的尺寸依赖性特性提供了进一步的物理见解,并强调了消光系数的理论值与实验值之间的差异,其中消光系数受角度相关的散射强度和所使用的测量技术的影响。











































 京公网安备 11010802027423号
京公网安备 11010802027423号