Applied Catalysis A: General ( IF 4.7 ) Pub Date : 2017-09-20 , DOI: 10.1016/j.apcata.2017.09.023 Stefan Ewald , Sebastian Standl , Olaf Hinrichsen
|
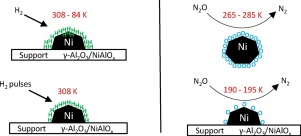
The Ni surface area is a key factor in catalytic performance of supported Ni catalysts employed in heterogeneously catalyzed reactions. For its characterization, transient measurement techniques are powerful but need a proper experimental design and a high level of accuracy. In this study, several transient methods, namely temperature programmed desorption of H2 (H2-TPD), pulsed H2 chemisorption and N2O chemisorption, were applied and evaluated for the characterization of Ni catalysts. Results were compared with those from static H2 chemisorption. Ni powder, as well as NiAlOx and Ni/γ-Al2O3 with varying Ni loadings, synthesized via precipitation and incipient wetness impregnation, respectively, were used as model catalysts. H2-TPD within the temperature range from 84 to 753 K is able to completely describe the interaction of H2 with supported Ni. However, a quantitative analysis of the specific Ni surface area based on H2-TPD is difficult whereas pulsed H2 chemisorption leads to comparable results as obtained from static measurements. Adsorption and pre-treatment conditions have a strong impact on the desorption spectra. Reversible morphologic changes of the Ni surface were revealed by H2-TPD when changing the gas atmosphere during pre‐treatment. N2O chemisorption occurs in three steps including fast oxygen uptake, growth of the oxide layer and subsequent layer thickening due to subsurface and bulk oxidation. A separation of surface and subsurface/bulk oxidation of NiAlOx and Ni/γ-Al2O3, both of which are readily oxidized even under mild conditions, is only achievable at temperatures between 190 and 195 K. In this temperature range, the ratio of adsorbed oxygen atoms, O, and the number of Ni surface atoms, NiS, (O/NiS) is 0.38 ± 0.07, which can be applied for specific Ni surface area determination. The experimental mode of N2O chemisorption (flow or titration mode) does not influence the extent of subsurface oxidation. The separation of surface and subsurface/bulk oxidation is more feasible in the case of Ni powder. Here, a suitable temperature for Ni surface area determination lies within 265 and 285 K with an O/NiS ratio of 0.96 ± 0.05.
中文翻译:

瞬态方法表征镍催化剂
Ni表面积是非均相催化反应中使用的负载型Ni催化剂的催化性能的关键因素。就其特性而言,瞬态测量技术功能强大,但需要适当的实验设计和高水平的准确性。在这项研究中,应用了几种瞬时方法,即H 2(H 2 -TPD)的程序升温脱附,脉冲H 2化学吸附和N 2 O化学吸附,并对Ni催化剂的表征进行了评估。将结果与静态H 2化学吸附的结果进行了比较。镍粉,以及NiAlO X和Ni /γ-Al系2 ö 3分别通过沉淀和初湿浸渍法合成的具有不同Ni负载量的Ni / Al用作模型催化剂。H 2 -TPD在84至753 K的温度范围内能够完全描述H 2与负载型Ni的相互作用。然而,基于H 2 -TPD的比Ni表面积的定量分析是困难的,而脉冲H 2化学吸附可得到从静态测量获得的可比结果。吸附和预处理条件对解吸光谱有很大影响。在预处理过程中改变气体气氛时,H 2 -TPD揭示了镍表面的可逆形态变化。N 2O化学吸附发生在三个步骤中,包括快速吸收氧气,氧化物层的生长以及由于地下和整体氧化而导致的随后的层增厚。表面和NiAlO的地下/散装氧化的分离X和Ni /γ-Al系2 ö 3,这两者的甚至在温和条件下容易被氧化,只是在190和195 K之间的温度下实现的。在该温度范围内,该所吸附的氧原子的比率O和Ni表面的原子数Ni S(O / Ni S)为0.38±0.07,可用于确定比表面积。N 2的实验模式O化学吸附(流动或滴定模式)不会影响地下氧化的程度。在镍粉的情况下,表面和次表面/本体氧化的分离更可行。在此,用于Ni表面积确定的合适温度在265和285K之间,O / Ni S比为0.96±0.05。











































 京公网安备 11010802027423号
京公网安备 11010802027423号