当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of Sulfanylated Difluoroalkenes: Electrophilic Difluoromethylidenation of Dithioesters with Difluorocarbene
Organic Letters ( IF 4.9 ) Pub Date : 2017-09-21 00:00:00 , DOI: 10.1021/acs.orglett.7b02222 Ryo Takayama 1 , Atsushi Yamada 1 , Kohei Fuchibe 1 , Junji Ichikawa 1
Organic Letters ( IF 4.9 ) Pub Date : 2017-09-21 00:00:00 , DOI: 10.1021/acs.orglett.7b02222 Ryo Takayama 1 , Atsushi Yamada 1 , Kohei Fuchibe 1 , Junji Ichikawa 1
Affiliation
|
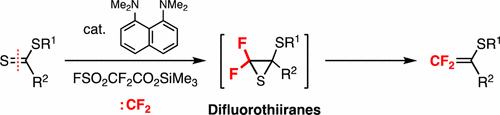
Electrophilic difluoromethylidenation of dithioesters was achieved in high yields via the reaction with difluorocarbene. When aryl or alkyl dithiocarboxylates were treated with trimethylsilyl 2,2-difluoro-2-fluorosulfonylacetate in the presence of 5 mol % of a Proton Sponge catalyst, the in situ generated difluorocarbene reacted with the dithioesters to afford 2-sulfanylated 1,1-difluoro-1-alkenes via difluorothiiranes. This reaction can be considered as an electrophilic counterpart of the Wittig-type difluoromethylidenation of carbonyl compounds with nucleophilic difluoromethylene ylides.
中文翻译:

磺酰化二氟烯烃的合成:二硫代卡宾与二氟卡宾的亲电二氟甲基亚甲基化
通过与二氟卡宾的反应,高收率地实现了二硫代酸酯的亲电子二氟甲基化。当在5摩尔%的质子海绵催化剂存在下,用三甲基甲硅烷基2,2-二氟-2-氟磺酰基乙酸酯处理芳基或二硫代羧酸烷基酯时,原位生成的二氟卡宾与二硫代酯反应生成2-磺酰化的1,1-二氟酯通过二氟噻喃生成-1-烯烃。该反应可视为羰基化合物的Wittig型二氟甲基化与亲核性二氟亚甲基酰基化物的亲电反应。
更新日期:2017-09-21
中文翻译:

磺酰化二氟烯烃的合成:二硫代卡宾与二氟卡宾的亲电二氟甲基亚甲基化
通过与二氟卡宾的反应,高收率地实现了二硫代酸酯的亲电子二氟甲基化。当在5摩尔%的质子海绵催化剂存在下,用三甲基甲硅烷基2,2-二氟-2-氟磺酰基乙酸酯处理芳基或二硫代羧酸烷基酯时,原位生成的二氟卡宾与二硫代酯反应生成2-磺酰化的1,1-二氟酯通过二氟噻喃生成-1-烯烃。该反应可视为羰基化合物的Wittig型二氟甲基化与亲核性二氟亚甲基酰基化物的亲电反应。











































 京公网安备 11010802027423号
京公网安备 11010802027423号