当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Tunable Cyclization Strategy for the Synthesis of Zizaene-, allo-Cedrane-, seco-Kaurane-, and seco-Atesane-Type Skeletons
Organic Letters ( IF 4.9 ) Pub Date : 2017-09-21 00:00:00 , DOI: 10.1021/acs.orglett.7b02610 Qianqian Yang 1 , Wenjing Ma 1 , Gaopeng Wang 1 , Wenli Bao 1 , Xiaoshu Dong 1 , Xuefeng Liang 1 , Lizhi Zhu 1 , Chi-Sing Lee 1
Organic Letters ( IF 4.9 ) Pub Date : 2017-09-21 00:00:00 , DOI: 10.1021/acs.orglett.7b02610 Qianqian Yang 1 , Wenjing Ma 1 , Gaopeng Wang 1 , Wenli Bao 1 , Xiaoshu Dong 1 , Xuefeng Liang 1 , Lizhi Zhu 1 , Chi-Sing Lee 1
Affiliation
|
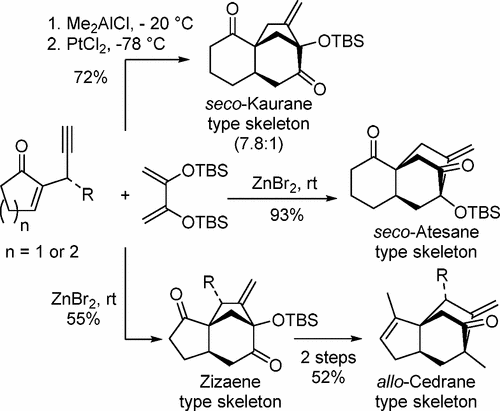
A versatile Lewis acid-mediated cyclization strategy has been developed for selectively establishing zizaene-, allo-cedrane-, seco-kaurane-, and seco-atesane-type skeletons. The zizaene- and seco-atesane-type skeletons can be obtained in a cascade manner, which involves Diels–Alder reaction of cyclic enones with bis-silyloxy dienes and carbocyclization of yne–enolates through Lewis acid dependent 5- or 6-exo-dig modes. This cyclization strategy was also employed for the core synthesis of tashironin.
中文翻译:

可调环化战略Zizaene-的合成,异体-Cedrane-,塞科-Kaurane-和塞科-Atesane型骨架
一种多功能路易斯酸介导的环化策略已经被开发用于选择性地建立zizaene-,同种异体-cedrane-,开环-kaurane-,和开环-atesane型骨架。的zizaene-和开环-atesane型骨架可以以级联的方式,它涉及与二甲硅烷氧基二烯和carbocyclization炔烯醇化物的通过路易斯酸依赖性5-或6-环烯酮的Diels-Alder反应来获得外切挖模式。这种环化策略也被用于tashironin的核心合成。
更新日期:2017-09-21
中文翻译:

可调环化战略Zizaene-的合成,异体-Cedrane-,塞科-Kaurane-和塞科-Atesane型骨架
一种多功能路易斯酸介导的环化策略已经被开发用于选择性地建立zizaene-,同种异体-cedrane-,开环-kaurane-,和开环-atesane型骨架。的zizaene-和开环-atesane型骨架可以以级联的方式,它涉及与二甲硅烷氧基二烯和carbocyclization炔烯醇化物的通过路易斯酸依赖性5-或6-环烯酮的Diels-Alder反应来获得外切挖模式。这种环化策略也被用于tashironin的核心合成。











































 京公网安备 11010802027423号
京公网安备 11010802027423号