当前位置:
X-MOL 学术
›
Chem. Phys. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
pH Dependent Domain Dynamics of HSA Controlled by Protein Based Crowding Agents
Chemical Physics Letters ( IF 2.8 ) Pub Date : 2017-09-21 , DOI: 10.1016/j.cplett.2017.09.034 Saikat Biswas , Sanjib K. Mukherjee , Harish Lal , Sandip Karmakar , Pramit K. Chowdhury
Chemical Physics Letters ( IF 2.8 ) Pub Date : 2017-09-21 , DOI: 10.1016/j.cplett.2017.09.034 Saikat Biswas , Sanjib K. Mukherjee , Harish Lal , Sandip Karmakar , Pramit K. Chowdhury
|
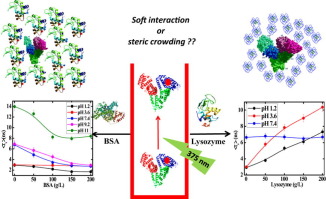
Here we have investigated the dynamics of domain I of human serum albumin (HSA) in presence of bovine serum albumin and lysozyme as crowders. 6-bromoacetyl-2-dimethylaminonaphthalene (BADAN) covalently attached to cysteine-34 of HSA was used as the solvation probe and changes in its solvation pattern in presence of the crowders were monitored as a function of pH. Lysozyme induced increased retardation of solvation while BSA brought about faster dynamics. Our observations reemphasize the importance of soft interactions even under conditions where repulsive charge-charge interactions dominate, thus reminding us of the enhanced level of complexity that the crowded milieu can possess.
中文翻译:

基于蛋白质的拥挤剂控制的HSA的pH依赖域动力学
在这里,我们研究了在牛血清白蛋白和溶菌酶作为拥挤剂存在下人血清白蛋白(HSA)的结构域I的动力学。共价连接到HSA的半胱氨酸34上的6-溴乙酰基-2-二甲基氨基萘(BADAN)被用作溶剂化探针,并在存在拥挤物的情况下监测其溶剂化模式随pH的变化。溶菌酶诱导增加的溶剂化阻滞,而BSA带来更快的动力学。我们的观察结果再次强调了软相互作用的重要性,即使在排斥性电荷-电荷相互作用占主导的条件下,也使我们想起了拥挤的环境可能具有的更高的复杂性。
更新日期:2017-09-21
中文翻译:

基于蛋白质的拥挤剂控制的HSA的pH依赖域动力学
在这里,我们研究了在牛血清白蛋白和溶菌酶作为拥挤剂存在下人血清白蛋白(HSA)的结构域I的动力学。共价连接到HSA的半胱氨酸34上的6-溴乙酰基-2-二甲基氨基萘(BADAN)被用作溶剂化探针,并在存在拥挤物的情况下监测其溶剂化模式随pH的变化。溶菌酶诱导增加的溶剂化阻滞,而BSA带来更快的动力学。我们的观察结果再次强调了软相互作用的重要性,即使在排斥性电荷-电荷相互作用占主导的条件下,也使我们想起了拥挤的环境可能具有的更高的复杂性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号