当前位置:
X-MOL 学术
›
ACS Chem. Neurosci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Comparison of α-Synuclein Fibril Inhibition by Four Different Amyloid Inhibitors
ACS Chemical Neuroscience ( IF 4.1 ) Pub Date : 2017-09-21 00:00:00 , DOI: 10.1021/acschemneuro.7b00261 Narendra Nath Jha 1 , Rakesh Kumar 1 , Rajlaxmi Panigrahi 1 , Ambuja Navalkar 1 , Dhiman Ghosh 1 , Shruti Sahay 1 , Mritunjoy Mondal 1 , Ashutosh Kumar 1 , Samir. K. Maji 1
ACS Chemical Neuroscience ( IF 4.1 ) Pub Date : 2017-09-21 00:00:00 , DOI: 10.1021/acschemneuro.7b00261 Narendra Nath Jha 1 , Rakesh Kumar 1 , Rajlaxmi Panigrahi 1 , Ambuja Navalkar 1 , Dhiman Ghosh 1 , Shruti Sahay 1 , Mritunjoy Mondal 1 , Ashutosh Kumar 1 , Samir. K. Maji 1
Affiliation
|
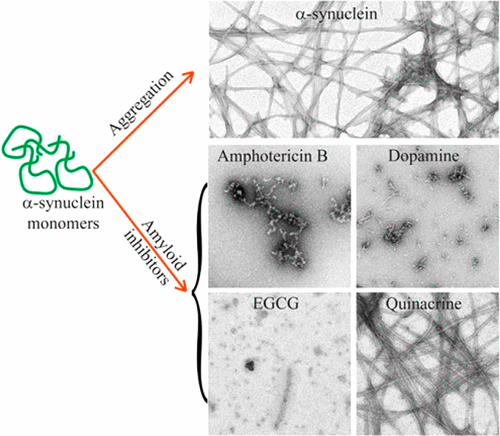
Aggregation of α-synuclein (α-Syn) into toxic oligomers and fibrils leads to Parkinson’s disease (PD) pathogenesis. Molecules that can inhibit the fibrillization and oligomerization of α-Syn have potential therapeutic value. Here, we studied four selective amyloid inhibitors: dopamine (Dopa), amphotericin-B (Amph), epigallocatechingallate (EGCG), and quinacrinedihydrochloride (Quin) for their effect on oligomerization, fibrillization, and preformed fibrils of α-Syn. The aggregation kinetics of α-Syn using ThT fluorescence and conformational transition by circular dichroism (CD) in the presence and absence of these four compounds suggest that, except Quin, the remaining three molecules inhibit α-Syn aggregation in a concentration dependent manner. Consistent with the aggregation kinetics data, the morphological study of aggregates formed in the presence of these compounds showed corresponding decrease in fibrillar size. The analysis of cell viability using MTT assay showed reduction in toxicity of α-Syn aggregates formed in the presence of these compounds, which also correlates with reduction of exposed hydrophobic surface as studied by ANS binding. Additionally, these inhibitors, except Quin, demonstrated reduction in size as well as the toxicity of oligomeric/fibrillar aggregates of α-Syn. The residue specific interaction to low molecular weight (LMW) species of α-Syn by 2D NMR study revealed that, the region and extent of binding are different for all these molecules. Furthermore, fibril-binding data using SPR suggested that there is no direct relationship between the binding affinity and fibril inhibition by these compounds. The present study suggests that sequence based interaction of small molecules with soluble α-Syn might dictate their inhibition or modulation capacity, which might be helpful in designing modulators of α-Syn aggregation.
中文翻译:

四种不同淀粉样蛋白抑制剂对α-突触核蛋白原纤维抑制作用的比较
α-突触核蛋白(α-Syn)聚集成有毒的寡聚物和原纤维会导致帕金森氏病(PD)的发病机理。可以抑制α-Syn的原纤维化和低聚的分子具有潜在的治疗价值。在这里,我们研究了四种选择性淀粉样蛋白抑制剂:多巴胺(Dopa),两性霉素B(Amph),表没食子儿茶酸酯(EGCG)和奎纳克林二盐酸盐(Quin)对α-Syn的低聚,原纤维化和原纤维形成的影响。在存在和不存在这四种化合物的情况下,使用ThT荧光和通过圆二色性(CD)进行构象转变的α-Syn聚集动力学表明,除Quin外,其余三个分子以浓度依赖性方式抑制α-Syn聚集。与聚集动力学数据一致,在这些化合物存在下形成的聚集体的形态学研究表明,原纤维尺寸相应减小。使用MTT分析对细胞活力进行的分析表明,在存在这些化合物的情况下形成的α-Syn聚集体的毒性降低,这也与ANS结合研究的暴露的疏水性表面的降低有关。此外,除Quin外,这些抑制剂均表现出α-Syn寡聚体/原纤维聚集体的尺寸减小以及毒性。通过2D NMR研究,残基与α-Syn低分子量(LMW)物种的特异性相互作用表明,所有这些分子的结合区域和结合程度均不同。此外,使用SPR的原纤维结合数据表明,这些化合物的结合亲和力与原纤维抑制之间没有直接关系。
更新日期:2017-09-21
中文翻译:

四种不同淀粉样蛋白抑制剂对α-突触核蛋白原纤维抑制作用的比较
α-突触核蛋白(α-Syn)聚集成有毒的寡聚物和原纤维会导致帕金森氏病(PD)的发病机理。可以抑制α-Syn的原纤维化和低聚的分子具有潜在的治疗价值。在这里,我们研究了四种选择性淀粉样蛋白抑制剂:多巴胺(Dopa),两性霉素B(Amph),表没食子儿茶酸酯(EGCG)和奎纳克林二盐酸盐(Quin)对α-Syn的低聚,原纤维化和原纤维形成的影响。在存在和不存在这四种化合物的情况下,使用ThT荧光和通过圆二色性(CD)进行构象转变的α-Syn聚集动力学表明,除Quin外,其余三个分子以浓度依赖性方式抑制α-Syn聚集。与聚集动力学数据一致,在这些化合物存在下形成的聚集体的形态学研究表明,原纤维尺寸相应减小。使用MTT分析对细胞活力进行的分析表明,在存在这些化合物的情况下形成的α-Syn聚集体的毒性降低,这也与ANS结合研究的暴露的疏水性表面的降低有关。此外,除Quin外,这些抑制剂均表现出α-Syn寡聚体/原纤维聚集体的尺寸减小以及毒性。通过2D NMR研究,残基与α-Syn低分子量(LMW)物种的特异性相互作用表明,所有这些分子的结合区域和结合程度均不同。此外,使用SPR的原纤维结合数据表明,这些化合物的结合亲和力与原纤维抑制之间没有直接关系。











































 京公网安备 11010802027423号
京公网安备 11010802027423号