Molecular Cell ( IF 14.5 ) Pub Date : 2017-09-19 , DOI: 10.1016/j.molcel.2017.08.009 Yuta Shinohara , Yohei M. Koyama , Maki Ukai-Tadenuma , Takatsugu Hirokawa , Masaki Kikuchi , Rikuhiro G. Yamada , Hideki Ukai , Hiroshi Fujishima , Takashi Umehara , Kazuki Tainaka , Hiroki R. Ueda
|
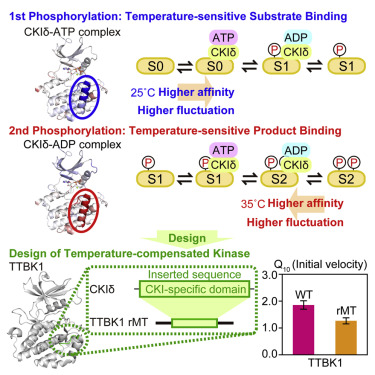
Temperature compensation is a striking feature of the circadian clock. Here we investigate biochemical mechanisms underlying temperature-compensated, CKIδ-dependent multi-site phosphorylation in mammals. We identify two mechanisms for temperature-insensitive phosphorylation at higher temperature: lower substrate affinity to CKIδ-ATP complex and higher product affinity to CKIδ-ADP complex. Inhibitor screening of ADP-dependent phosphatase activity of CKIδ identified aurintricarboxylic acid (ATA) as a temperature-sensitive kinase activator. Docking simulation of ATA and mutagenesis experiment revealed K224D/K224E mutations in CKIδ that impaired product binding and temperature-compensated primed phosphorylation. Importantly, K224D mutation shortens behavioral circadian rhythms and changes the temperature dependency of SCN’s circadian period. Interestingly, temperature-compensated phosphorylation was evolutionary conserved in yeast. Molecular dynamics simulation and X-ray crystallography demonstrate that an evolutionally conserved CKI-specific domain around K224 can provide a structural basis for temperature-sensitive substrate and product binding. Surprisingly, this domain can confer temperature compensation on a temperature-sensitive TTBK1. These findings suggest the temperature-sensitive substrate- and product-binding mechanisms underlie temperature compensation.
中文翻译:

温度敏感的底物和产物结合是时钟中温度补偿的磷酸化的基础
温度补偿是生物钟的显着特征。在这里,我们研究哺乳动物中温度补偿的,依赖CKIδ的多位磷酸化的生化机制。我们确定了高温下对温度不敏感的磷酸化的两种机制:对CKIδ-ATP复合物较低的底物亲和力和对CKIδ-ADP复合物较高的产物亲和力。对CKIδ的ADP依赖性磷酸酶活性的抑制剂筛选确定了金三羧酸(ATA)是温度敏感的激酶激活剂。ATA的对接模拟和诱变实验表明,CKIδ中的K224D / K224E突变会损害产物结合和温度补偿的引发磷酸化。重要的是,K224D突变缩短了行为昼夜节律并改变了SCN昼夜节律的温度依赖性。有趣的是,温度补偿的磷酸化在酵母中是进化保守的。分子动力学模拟和X射线晶体学表明,K224周围进化保守的CKI特定域可以为温度敏感的底物和产物结合提供结构基础。出乎意料的是,该域可以对温度敏感的TTBK1赋予温度补偿。这些发现表明,温度敏感的底物和产物结合机理是温度补偿的基础。该域可以对温度敏感的TTBK1进行温度补偿。这些发现表明,温度敏感的底物和产物结合机理是温度补偿的基础。该域可以对温度敏感的TTBK1进行温度补偿。这些发现表明,温度敏感的底物和产物结合机理是温度补偿的基础。











































 京公网安备 11010802027423号
京公网安备 11010802027423号