Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Biomimetic Magnetosomes as Versatile Artificial Antigen-Presenting Cells to Potentiate T-Cell-Based Anticancer Therapy
ACS Nano ( IF 15.8 ) Pub Date : 2017-09-20 00:00:00 , DOI: 10.1021/acsnano.7b04955 Qianmei Zhang 1 , Wei Wei 2 , Peilin Wang 1 , Liping Zuo 1 , Feng Li 1 , Jin Xu 1 , Xiaobo Xi 2 , Xiaoyong Gao 2 , Guanghui Ma 2 , Hai-yan Xie 1
ACS Nano ( IF 15.8 ) Pub Date : 2017-09-20 00:00:00 , DOI: 10.1021/acsnano.7b04955 Qianmei Zhang 1 , Wei Wei 2 , Peilin Wang 1 , Liping Zuo 1 , Feng Li 1 , Jin Xu 1 , Xiaobo Xi 2 , Xiaoyong Gao 2 , Guanghui Ma 2 , Hai-yan Xie 1
Affiliation
|
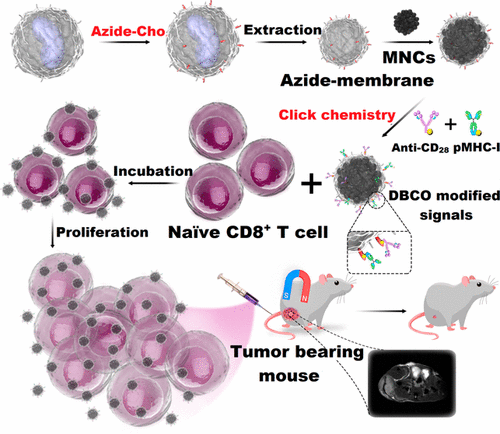
Adoptive T-cell transfer for cancer therapy relies on both effective ex vivo T-cell expansion and in vivo targeting performance. One promising but challenging method for accomplishing this purpose is to construct multifunctional artificial antigen-presenting cells (aAPCs). We herein developed biomimetic magnetosomes as versatile aAPCs, wherein magnetic nanoclusters were coated with azide-engineered leucocyte membranes and then decorated with T-cell stimuli through copper-free click chemistry. These nano aAPCs not only exhibited high performance for antigen-specific cytotoxic T-cell (CTL) expansion and stimulation but also visually and effectively guided reinfused CTLs to tumor tissues through magnetic resonance imaging and magnetic control. The persisting T cells were able to delay tumor growth in a murine lymphoma model, while the systemic toxicity was not notable. These results together demonstrated the excellent potential of this “one-but-all” aAPC platform for T-cell-based anticancer immunotherapy.
中文翻译:

仿生磁小体作为多功能人工抗原呈递细胞,可增强基于T细胞的抗癌治疗
用于癌症治疗的过继性T细胞转移依赖于有效的离体T细胞扩增和体内定位效果。实现该目的的一种有希望但具有挑战性的方法是构建多功能人工抗原呈递细胞(aAPC)。我们在此开发了仿生的磁小体,作为通用的aAPC,其中磁性纳米簇被叠氮化物工程化的白细胞膜覆盖,然后通过无铜点击化学用T细胞刺激物修饰。这些纳米aAPC不仅表现出对抗原特异性细胞毒性T细胞(CTL)扩增和刺激的高性能,而且还通过磁共振成像和磁控制在视觉上有效地将重新注入的CTL引导至肿瘤组织。在鼠淋巴瘤模型中,持久的T细胞能够延迟肿瘤的生长,而全身毒性并不显着。
更新日期:2017-09-20
中文翻译:

仿生磁小体作为多功能人工抗原呈递细胞,可增强基于T细胞的抗癌治疗
用于癌症治疗的过继性T细胞转移依赖于有效的离体T细胞扩增和体内定位效果。实现该目的的一种有希望但具有挑战性的方法是构建多功能人工抗原呈递细胞(aAPC)。我们在此开发了仿生的磁小体,作为通用的aAPC,其中磁性纳米簇被叠氮化物工程化的白细胞膜覆盖,然后通过无铜点击化学用T细胞刺激物修饰。这些纳米aAPC不仅表现出对抗原特异性细胞毒性T细胞(CTL)扩增和刺激的高性能,而且还通过磁共振成像和磁控制在视觉上有效地将重新注入的CTL引导至肿瘤组织。在鼠淋巴瘤模型中,持久的T细胞能够延迟肿瘤的生长,而全身毒性并不显着。











































 京公网安备 11010802027423号
京公网安备 11010802027423号