当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Stereoselective Michael—Mannich Annelation Strategy for the Construction of Novel Tetrahydrocarbazoles
Organic Letters ( IF 4.9 ) Pub Date : 2017-09-20 00:00:00 , DOI: 10.1021/acs.orglett.7b02323 David R. Williams 1 , Seth A. Bawel 1 , Richard N. Schaugaard 1
Organic Letters ( IF 4.9 ) Pub Date : 2017-09-20 00:00:00 , DOI: 10.1021/acs.orglett.7b02323 David R. Williams 1 , Seth A. Bawel 1 , Richard N. Schaugaard 1
Affiliation
|
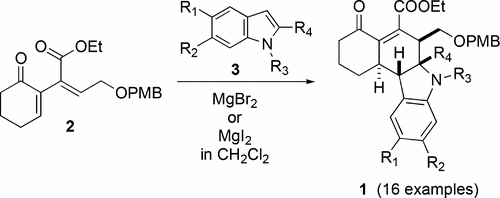
A novel annelation strategy has been devised for stereoselective synthesis of tetrahydrocarbazoles. The pathway features a regio- and stereocontrolled condensation of indole and its substituted derivatives with electron-deficient 1,3-dienes via a Michael–Mannich reaction sequence. An extension of this method to include cross-conjugated allenes as substrates also results in a Michael–Mannich–Michael cascade, incorporating 2 equiv of indole with increasing product complexity. The formal 4π + 2π cyclization describes a concise route to polycyclic alkaloids of this family.
中文翻译:

建立新型四氢咔唑的立体选择性Michael-Mannich退火策略
已经设计出一种新颖的去核策略用于四氢咔唑的立体选择性合成。该途径的特征是通过迈克尔-曼尼希(Michael-Mannich)反应序列,使吲哚及其取代的衍生物与电子不足的1,3-二烯进行区域和立体控制的缩合。将该方法扩展为包括交叉共轭的烯类作为底物,还导致了Michael–Mannich–Michael级联反应,其中引入了2当量的吲哚,并增加了产品的复杂性。正式的4π+2π环化反应为该家族的多环生物碱提供了一条简洁的途径。
更新日期:2017-09-20
中文翻译:

建立新型四氢咔唑的立体选择性Michael-Mannich退火策略
已经设计出一种新颖的去核策略用于四氢咔唑的立体选择性合成。该途径的特征是通过迈克尔-曼尼希(Michael-Mannich)反应序列,使吲哚及其取代的衍生物与电子不足的1,3-二烯进行区域和立体控制的缩合。将该方法扩展为包括交叉共轭的烯类作为底物,还导致了Michael–Mannich–Michael级联反应,其中引入了2当量的吲哚,并增加了产品的复杂性。正式的4π+2π环化反应为该家族的多环生物碱提供了一条简洁的途径。











































 京公网安备 11010802027423号
京公网安备 11010802027423号