当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Remote Migratory Cross-Electrophile Coupling and Olefin Hydroarylation Reactions Enabled by in Situ Generation of NiH
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-09-20 00:00:00 , DOI: 10.1021/jacs.7b08064 Fenglin Chen 1 , Ke Chen 1 , Yao Zhang 1 , Yuli He 1 , Yi-Ming Wang 2 , Shaolin Zhu 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-09-20 00:00:00 , DOI: 10.1021/jacs.7b08064 Fenglin Chen 1 , Ke Chen 1 , Yao Zhang 1 , Yuli He 1 , Yi-Ming Wang 2 , Shaolin Zhu 1
Affiliation
|
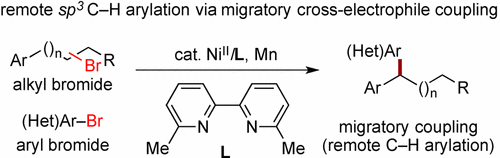
A highly efficient strategy for remote reductive cross-electrophile coupling has been developed through the ligand-controlled nickel migration/arylation. This general protocol allows the use of abundant and bench-stable alkyl bromides and aryl bromides for the synthesis of a wide range of structurally diverse 1,1-diarylalkanes in excellent yields and high regioselectivities under mild conditions. We also demonstrated that alkyl bromide could be replaced by the proposed olefin intermediate while using n-propyl bromide/Mn0 as a potential hydride source.
中文翻译:

NiH的原位产生远程迁移性亲电子偶联和烯烃氢化反应
通过配体控制的镍迁移/芳构化,已经开发出一种用于远程还原性交亲电试剂偶联的高效策略。该通用方案允许使用大量稳定的烷基溴化物和芳基溴化物,在温和条件下以优异的收率和高区域选择性合成各种结构多样的1,1-二芳基烷烃。我们还证明,在使用正丙基溴/ Mn 0作为潜在的氢化物源的同时,烷基溴可以被提议的烯烃中间体替代。
更新日期:2017-09-20
中文翻译:

NiH的原位产生远程迁移性亲电子偶联和烯烃氢化反应
通过配体控制的镍迁移/芳构化,已经开发出一种用于远程还原性交亲电试剂偶联的高效策略。该通用方案允许使用大量稳定的烷基溴化物和芳基溴化物,在温和条件下以优异的收率和高区域选择性合成各种结构多样的1,1-二芳基烷烃。我们还证明,在使用正丙基溴/ Mn 0作为潜在的氢化物源的同时,烷基溴可以被提议的烯烃中间体替代。











































 京公网安备 11010802027423号
京公网安备 11010802027423号