当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Role of Li Concentration and the SEI Layer in Enabling High Performance Li Metal Electrodes Using a Phosphonium Bis(fluorosulfonyl)imide Ionic Liquid
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2017-09-20 00:00:00 , DOI: 10.1021/acs.jpcc.7b01929 Gaetan M. A. Girard 1 , Matthias Hilder 1 , Donato Nucciarone 2 , Kristina Whitbread 2 , Serguei Zavorine 2 , Michael Moser 2 , Maria Forsyth 1 , Douglas R. MacFarlane 3 , Patrick C. Howlett 1
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2017-09-20 00:00:00 , DOI: 10.1021/acs.jpcc.7b01929 Gaetan M. A. Girard 1 , Matthias Hilder 1 , Donato Nucciarone 2 , Kristina Whitbread 2 , Serguei Zavorine 2 , Michael Moser 2 , Maria Forsyth 1 , Douglas R. MacFarlane 3 , Patrick C. Howlett 1
Affiliation
|
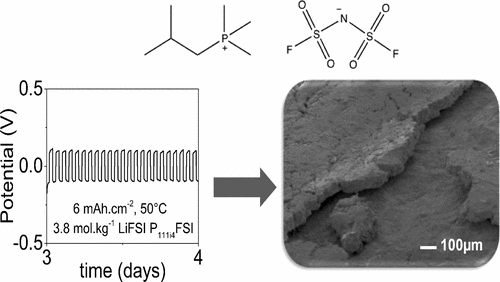
In this study the performance of the lithium (Li) anode is characterized in two alternative ionic liquid electrolytes: (i) a solution of 0.5 mol·kg–1 of lithium bis(fluorosulfonyl)imide (LiFSI) in trimethyl(isobutyl)phosphonium FSI (P111i4FSI) and (ii) an equimolar mixture of these two salts, effectively an inorganic–organic mixture IL. We have investigated the formation of the solid electrolyte interphase (SEI) at the lithium electrode and its influence on the polarization potential, the electrode surface impedance and deposition morphologies. Lithium metal cycling is revealed to be significantly more stable in the electrolyte with high lithium salt concentration due to the creation of a more uniform SEI. Stable and effective cycling was demonstrated at high applied currents (up to 12 mA·cm–2) with large areal capacities being transferred with each polarization cycle (up to 6 mAh·cm–2 at 50 °C). An average Coulombic efficiency of not less than 99.2% was demonstrated under these conditions and SEM observations of the cycled electrode surfaces show a uniform and compact deposit. Combined with spectroscopic characterization of the electrolyte and electrode surface, these observations indicate a role for the speciation and transport properties of these high concentration ionic liquid electrolytes in modifiying the physicochemical properties of the SEI which result in enhanced cycling performance of the Li metal electrode.
中文翻译:

Li浓度和SEI层在使用双(氟磺酰基)酰亚胺离子液体制造高性能Li金属电极中的作用
在这项研究中,使用两种替代性离子液体电解质来表征锂(Li)阳极的性能:(i)0.5 mol·kg –1的双(氟磺酰基)酰亚胺锂(LiFSI)在三甲基(异丁基)phosph FSI中的溶液(P 111i4FSI)和(ii)这两种盐的等摩尔混合物,实际上是无机-有机混合物IL。我们已经研究了在锂电极上形成的固态电解质中间相(SEI)及其对极化电势,电极表面阻抗和沉积形态的影响。由于产生更均匀的SEI,锂金属循环在具有高锂盐浓度的电解质中显示出明显更稳定的状态。在高施加电流(高达12 mA·cm –2)下表现出了稳定有效的循环,每个极化循环(高达6 mAh·cm –2)传递了大的面容量在50°C时)。在这些条件下,平均库仑效率不低于99.2%,循环电极表面的SEM观察显示均匀而致密的沉积。结合电解质和电极表面的光谱表征,这些观察结果表明这些高浓度离子液体电解质的形态和传输性质在修饰SEI的理化性质方面起着作用,从而提高了Li金属电极的循环性能。
更新日期:2017-09-20
中文翻译:

Li浓度和SEI层在使用双(氟磺酰基)酰亚胺离子液体制造高性能Li金属电极中的作用
在这项研究中,使用两种替代性离子液体电解质来表征锂(Li)阳极的性能:(i)0.5 mol·kg –1的双(氟磺酰基)酰亚胺锂(LiFSI)在三甲基(异丁基)phosph FSI中的溶液(P 111i4FSI)和(ii)这两种盐的等摩尔混合物,实际上是无机-有机混合物IL。我们已经研究了在锂电极上形成的固态电解质中间相(SEI)及其对极化电势,电极表面阻抗和沉积形态的影响。由于产生更均匀的SEI,锂金属循环在具有高锂盐浓度的电解质中显示出明显更稳定的状态。在高施加电流(高达12 mA·cm –2)下表现出了稳定有效的循环,每个极化循环(高达6 mAh·cm –2)传递了大的面容量在50°C时)。在这些条件下,平均库仑效率不低于99.2%,循环电极表面的SEM观察显示均匀而致密的沉积。结合电解质和电极表面的光谱表征,这些观察结果表明这些高浓度离子液体电解质的形态和传输性质在修饰SEI的理化性质方面起着作用,从而提高了Li金属电极的循环性能。











































 京公网安备 11010802027423号
京公网安备 11010802027423号