当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structure and Reactivity of a Model Oxide Supported Silver Nanocluster Catalyst Studied by Near Ambient Pressure X-ray Photoelectron Spectroscopy
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2017-09-20 00:00:00 , DOI: 10.1021/acs.jpcc.7b05818 Michael Wagstaffe 1 , Hadeel Hussain 2 , Matthew J. Acres 2 , Rosemary Jones 3 , Karen L. Syres 4 , Andrew G. Thomas 2, 3
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2017-09-20 00:00:00 , DOI: 10.1021/acs.jpcc.7b05818 Michael Wagstaffe 1 , Hadeel Hussain 2 , Matthew J. Acres 2 , Rosemary Jones 3 , Karen L. Syres 4 , Andrew G. Thomas 2, 3
Affiliation
|
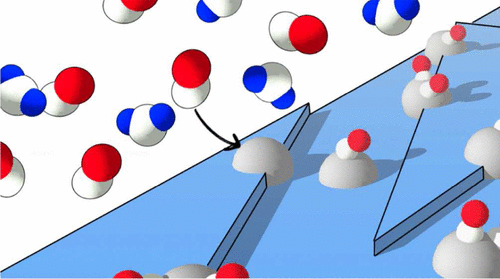
The photocatalytic activity of anatase TiO2 decorated with metal clusters has been widely documented, but the nature of the metal–metal oxide interface and reaction intermediates in catalytic processes are still not well understood. This in part is due to the fact that use of photoelectron spectroscopy to deduce the surface chemistry of catalytic systems has long been hampered by the huge pressure difference between real-world operation and the requirement of high vacuum for electron detection. Here, the in situ growth of silver nanoparticles on a model metal-oxide catalyst support and their reactivity with a CO/H2O gas mixture has been investigated in detail. Using synchrotron X-ray photoelectron spectroscopy, near-ambient pressure X-ray photoelectron spectroscopy, and scanning tunneling microscopy, the interaction of Ag with the anatase TiO2 surface leads to metal-surface charge transfer and low mobility of Ag on the surface. Upon exposure to a 1.5 mbar CO/1.5 mbar H2O gas mixture, partial oxidation of the Ag clusters is observed. There is also evidence suggesting that a Ag–carbonyl species is formed during exposure of the Ag/TiO2 surface to a CO/H2O gas mixture.
中文翻译:

近环境压力X射线光电子能谱研究模型氧化物负载的银纳米团簇催化剂的结构和反应活性
装饰有金属团簇的锐钛矿型TiO 2的光催化活性已得到广泛文献记载,但是金属-金属氧化物界面和催化过程中的反应中间体的性质仍不为人所知。这部分是由于以下事实:长期以来,由于实际操作与电子检测需要高真空之间的巨大压差,使用光电子能谱推论催化体系的表面化学一直受到阻碍。在这里,银纳米颗粒在模型金属氧化物催化剂载体上的原位生长及其与CO / H 2的反应性已对O混合气体进行了详细研究。使用同步加速器X射线光电子能谱,近环境压力X射线光电子能谱和扫描隧道显微镜,Ag与锐钛矿型TiO 2表面的相互作用导致金属表面电荷转移和表面上Ag的低迁移率。暴露于1.5 mbar CO / 1.5 mbar H 2 O气体混合物中,观察到Ag团簇的部分氧化。也有证据表明,在将Ag / TiO 2表面暴露于CO / H 2 O气体混合物期间会形成Ag-羰基物质。
更新日期:2017-09-20
中文翻译:

近环境压力X射线光电子能谱研究模型氧化物负载的银纳米团簇催化剂的结构和反应活性
装饰有金属团簇的锐钛矿型TiO 2的光催化活性已得到广泛文献记载,但是金属-金属氧化物界面和催化过程中的反应中间体的性质仍不为人所知。这部分是由于以下事实:长期以来,由于实际操作与电子检测需要高真空之间的巨大压差,使用光电子能谱推论催化体系的表面化学一直受到阻碍。在这里,银纳米颗粒在模型金属氧化物催化剂载体上的原位生长及其与CO / H 2的反应性已对O混合气体进行了详细研究。使用同步加速器X射线光电子能谱,近环境压力X射线光电子能谱和扫描隧道显微镜,Ag与锐钛矿型TiO 2表面的相互作用导致金属表面电荷转移和表面上Ag的低迁移率。暴露于1.5 mbar CO / 1.5 mbar H 2 O气体混合物中,观察到Ag团簇的部分氧化。也有证据表明,在将Ag / TiO 2表面暴露于CO / H 2 O气体混合物期间会形成Ag-羰基物质。











































 京公网安备 11010802027423号
京公网安备 11010802027423号