Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
New Tethered Phospholipid Bilayers Integrating Functional G-Protein-Coupled Receptor Membrane Proteins
Langmuir ( IF 3.7 ) Pub Date : 2017-09-20 00:00:00 , DOI: 10.1021/acs.langmuir.7b01636 Meriem Chadli 1, 2 , Samuel Rebaud 1 , Ofelia Maniti 1 , Bruno Tillier 2 , Sandra Cortès 2 , Agnès Girard-Egrot 1
Langmuir ( IF 3.7 ) Pub Date : 2017-09-20 00:00:00 , DOI: 10.1021/acs.langmuir.7b01636 Meriem Chadli 1, 2 , Samuel Rebaud 1 , Ofelia Maniti 1 , Bruno Tillier 2 , Sandra Cortès 2 , Agnès Girard-Egrot 1
Affiliation
|
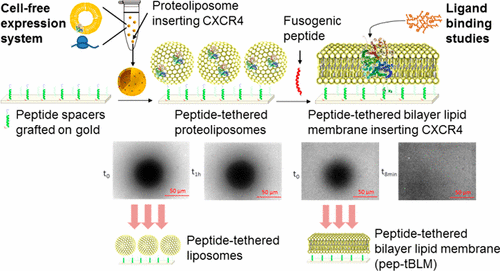
Membrane proteins exhibiting extra- and intracellular domains require an adequate near-native lipid platform for their functional reconstitution. With this aim, we developed a new technology enabling the formation of a peptide-tethered bilayer lipid membrane (pep-tBLM), a lipid bilayer grafted onto peptide spacers, by way of a metal–chelate interaction. To this end, we designed an original peptide spacer derived from the natural α-laminin thiopeptide (P19) possessing a cysteine residue in the N-terminal extremity for grafting onto gold and a C-terminal extremity modified by four histidine residues (P19-4H). In the presence of nickel, the use of this anchor allowed us to bind liposomes of variable compositions containing a 2% molar ratio of a chelating lipid, 1,2-dioleoyl-sn-glycero-3-[(N-(5-amino-1-carboxypentyl)iminodiacetic acid)succinyl] so-called DOGS-NTA, and to form the planar bilayer by triggering liposome fusion by an α-helical (AH) peptide derived from the N-terminus of the hepatitis C virus NS5A protein. The formation of pep-tBLMs was characterized by surface plasmon resonance imaging (SPRi), and their continuity, fluidity, and homogeneity were demonstrated by fluorescence recovery after photobleaching (FRAP), with a diffusion coefficient of 2.5 × 10–7 cm2/s, and atomic force microscopy (AFM). By using variable lipid compositions including phosphatidylcholine (PC), phosphatidylserine (PS), phosphatidylethanolamine (PE), phosphatidylinositol 4,5-bisphosphate (PIP2), sphingomyelin (SM), phosphatidic acid (PA), and cholesterol (Chol) in various ratios, we show that the membrane can be formed independently from the lipid composition. We made the most of this advantage to reincorporate a transmembrane protein in an adapted complex lipid composition to ensure its functional reinsertion. For this purpose, a cell-free expression system was used to produce proteoliposomes expressing the functional C-X-C motif chemokine receptor 4 (CXCR4), a seven-transmembrane protein belonging to the large superfamily of G-protein-coupled receptors (GPCRs). We succeeded in reinserting CXCR4 in pep-tBLMs formed on P19-4H by the fusion of tethered proteoliposomes. AFM and FRAP characterization allowed us to show that pep-tBLMs inserting CXCR4 remained fluid, homogeneous, and continuous. The value of the diffusion coefficient determined in the presence of reinserted CXCR4 was 2 × 10–7 cm2/s. Ligand binding assays using a synthetic CXCR4 antagonist, T22 ([Tyr5,12, Lys7]-polyphemusin II), revealed that CXCR4 can be reinserted in pep-tBLMs with functional folding and orientation. This new approach represents a method of choice for investigating membrane protein reincorporation and a promising way of creating a new generation of membrane biochips adapted for screening agonists or antagonists of transmembrane proteins.
中文翻译:

整合功能性G蛋白偶联受体膜蛋白的新型束缚的磷脂双层。
表现出胞外和胞内结构域的膜蛋白需要适当的近天然脂质平台来进行功能重建。为此,我们开发了一种新技术,该技术能够通过金属-螯合物的相互作用形成肽连接的双层脂质膜(pep-tBLM),这是一种嫁接到肽间隔基上的脂质双层。为此,我们设计了一个天然的肽间隔物,该肽间隔物源自天然的α-laminin硫肽(P19),该肽在N末端具有一个半胱氨酸残基,可以嫁接到金上,而C末端由四个组氨酸残基修饰(P19-4H )。在镍的存在下,使用这种锚固剂使我们能够结合可变组成的脂质体,该脂质体包含2%摩尔比的螯合脂质1,2-二油酰基-sn-甘油-3-[[ N-(5-氨基-1-羧基戊基)亚氨基二乙酸)琥珀酰基],称为DOGS-NTA,并通过由肝炎N末端衍生的α-螺旋(AH)肽触发脂质体融合而形成平面双层C病毒NS5A蛋白。pep-tBLM的形成通过表面等离子体共振成像(SPRi)表征,其连续性,流动性和均一性通过光漂白后的荧光恢复(FRAP)证明,扩散系数为2.5×10 –7 cm 2 / s ,以及原子力显微镜(AFM)。通过使用可变的脂质成分,包括磷脂酰胆碱(PC),磷脂酰丝氨酸(PS),磷脂酰乙醇胺(PE),磷脂酰肌醇4,5-双磷酸酯(PIP 2),鞘磷脂(SM),磷脂酸(PA)和胆固醇(Chol)的各种比例,我们证明该膜可以独立于脂质组成而形成。我们充分利用了这一优势,可以将跨膜蛋白重新整合到适应的复杂脂质组合物中,以确保其功能重新插入。为此目的,使用无细胞表达系统生产表达功能性CXC基序趋化因子受体4(CXCR4)的蛋白脂质体,CXC基序趋化因子受体4是七大跨膜蛋白,属于G蛋白偶联受体(GPCR)的大超家族。我们通过链状蛋白脂质体的融合成功地将CXCR4重新插入P19-4H上形成的pep-tBLM中。原子力显微镜和FRAP表征使我们能够证明插入CXCR4的pep-tBLM保持流动,均匀和连续。–7厘米2 /秒。使用合成的CXCR4拮抗剂T22([Tyr5,12,Lys7]-多phemusin II)进行配体结合测定,结果表明CXCR4可以重新插入具有功能性折叠和方向的pep-tBLM中。这种新方法代表了研究膜蛋白重新掺入的一种选择方法,并且是创建适用于筛选跨膜蛋白激动剂或拮抗剂的新一代膜生物芯片的有前途的方式。
更新日期:2017-09-20
中文翻译:

整合功能性G蛋白偶联受体膜蛋白的新型束缚的磷脂双层。
表现出胞外和胞内结构域的膜蛋白需要适当的近天然脂质平台来进行功能重建。为此,我们开发了一种新技术,该技术能够通过金属-螯合物的相互作用形成肽连接的双层脂质膜(pep-tBLM),这是一种嫁接到肽间隔基上的脂质双层。为此,我们设计了一个天然的肽间隔物,该肽间隔物源自天然的α-laminin硫肽(P19),该肽在N末端具有一个半胱氨酸残基,可以嫁接到金上,而C末端由四个组氨酸残基修饰(P19-4H )。在镍的存在下,使用这种锚固剂使我们能够结合可变组成的脂质体,该脂质体包含2%摩尔比的螯合脂质1,2-二油酰基-sn-甘油-3-[[ N-(5-氨基-1-羧基戊基)亚氨基二乙酸)琥珀酰基],称为DOGS-NTA,并通过由肝炎N末端衍生的α-螺旋(AH)肽触发脂质体融合而形成平面双层C病毒NS5A蛋白。pep-tBLM的形成通过表面等离子体共振成像(SPRi)表征,其连续性,流动性和均一性通过光漂白后的荧光恢复(FRAP)证明,扩散系数为2.5×10 –7 cm 2 / s ,以及原子力显微镜(AFM)。通过使用可变的脂质成分,包括磷脂酰胆碱(PC),磷脂酰丝氨酸(PS),磷脂酰乙醇胺(PE),磷脂酰肌醇4,5-双磷酸酯(PIP 2),鞘磷脂(SM),磷脂酸(PA)和胆固醇(Chol)的各种比例,我们证明该膜可以独立于脂质组成而形成。我们充分利用了这一优势,可以将跨膜蛋白重新整合到适应的复杂脂质组合物中,以确保其功能重新插入。为此目的,使用无细胞表达系统生产表达功能性CXC基序趋化因子受体4(CXCR4)的蛋白脂质体,CXC基序趋化因子受体4是七大跨膜蛋白,属于G蛋白偶联受体(GPCR)的大超家族。我们通过链状蛋白脂质体的融合成功地将CXCR4重新插入P19-4H上形成的pep-tBLM中。原子力显微镜和FRAP表征使我们能够证明插入CXCR4的pep-tBLM保持流动,均匀和连续。–7厘米2 /秒。使用合成的CXCR4拮抗剂T22([Tyr5,12,Lys7]-多phemusin II)进行配体结合测定,结果表明CXCR4可以重新插入具有功能性折叠和方向的pep-tBLM中。这种新方法代表了研究膜蛋白重新掺入的一种选择方法,并且是创建适用于筛选跨膜蛋白激动剂或拮抗剂的新一代膜生物芯片的有前途的方式。











































 京公网安备 11010802027423号
京公网安备 11010802027423号