当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electrochemical Induced Calcium Phosphate Precipitation: Importance of Local pH
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2017-09-20 00:00:00 , DOI: 10.1021/acs.est.7b03909 Yang Lei 1, 2 , Bingnan Song 1, 2 , Renata D. van der Weijden 1, 2 , Michel Saakes 1 , Cees J. N. Buisman 1, 2
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2017-09-20 00:00:00 , DOI: 10.1021/acs.est.7b03909 Yang Lei 1, 2 , Bingnan Song 1, 2 , Renata D. van der Weijden 1, 2 , Michel Saakes 1 , Cees J. N. Buisman 1, 2
Affiliation
|
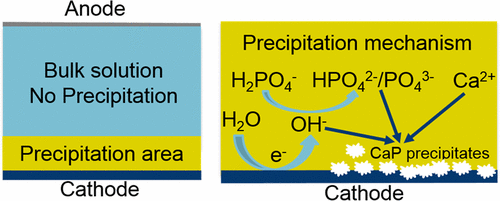
Phosphorus (P) is an essential nutrient for living organisms and cannot be replaced or substituted. In this paper, we present a simple yet efficient membrane free electrochemical system for P removal and recovery as calcium phosphate (CaP). This method relies on in situ formation of hydroxide ions by electro mediated water reduction at a titanium cathode surface. The in situ raised pH at the cathode provides a local environment where CaP will become highly supersaturated. Therefore, homogeneous and heterogeneous nucleation of CaP occurs near and at the cathode surface. Because of the local high pH, the P removal behavior is not sensitive to bulk solution pH and therefore, efficient P removal was observed in three studied bulk solutions with pH of 4.0 (56.1%), 8.2 (57.4%), and 10.0 (48.4%) after 24 h of reaction time. While P removal efficiencies are not generally affected by bulk solution pH, the chemical-physical properties of CaP solids collected on the cathode are still related to bulk solution pH, as confirmed by structure characterizations. High initial solution pH promotes the formation of more crystalline products with relatively high Ca/P molar ratio. The Ca/P molar ratio increases from 1.30 (pH 4.0) to 1.38 (pH 8.2) and further increases to 1.55 (pH 10.0). The formation of CaP precipitates was a typical crystallization process, with an amorphous phase formed at the initial stage which then transforms to the most stable crystal phase, hydroxyapatite, which is inferred from the increased Ca/P molar ratio from 1.38 (day 1) to the theoretical 1.76 (day 11) and by the formation of needle-like crystals. Finally, we demonstrated the efficiency of this system for real wastewater. This, together with the fact that the electrochemical method can work at low bulk pH, without dosing chemicals and a need for a separation process, highlights the potential application of the electrochemical method for P removal and recovery.
中文翻译:

电化学诱导的磷酸钙沉淀:局部pH的重要性
磷(P)是生物体必不可少的营养素,不能被替代或替代。在本文中,我们提出了一种简单而有效的无膜电化学系统,用于除磷和回收磷酸钙(CaP)。该方法依赖于通过电介导的水还原在钛阴极表面上原位形成氢氧根离子。阴极处的原位升高的pH值提供了CaP将变得高度过饱和的局部环境。因此,CaP的均匀和不均匀成核发生在阴极表面附近和阴极表面。由于局部pH高,P的去除行为对本体溶液的pH值不敏感,因此,在三种研究的pH分别为4.0(56.1%),8.2(57.4%)和10.0(48.4)的本体溶液中均观察到了有效的P去除。反应时间24小时后)。尽管脱磷效率通常不受本体溶液pH的影响,但通过结构表征证实,收集在阴极上的CaP固体的化学物理性质仍与本体溶液pH有关。较高的初始溶液pH值会促进形成具有较高Ca / P摩尔比的更多结晶产物。Ca / P摩尔比从1.30(pH 4.0)增加到1.38(pH 8.2),然后进一步增加到1.55(pH 10.0)。CaP沉淀物的形成是典型的结晶过程,在初始阶段形成无定形相,然后转变为最稳定的结晶相羟基磷灰石,这是由Ca / P摩尔比从1.38(第1天)提高至理论上为1.76(第11天)并由针状晶体形成。最后,我们证明了该系统对实际废水的效率。这与电化学方法可以在低总体pH值下工作而无需添加化学药品以及不需要分离过程的事实一起,突出了电化学方法在P去除和回收中的潜在应用。
更新日期:2017-09-20
中文翻译:

电化学诱导的磷酸钙沉淀:局部pH的重要性
磷(P)是生物体必不可少的营养素,不能被替代或替代。在本文中,我们提出了一种简单而有效的无膜电化学系统,用于除磷和回收磷酸钙(CaP)。该方法依赖于通过电介导的水还原在钛阴极表面上原位形成氢氧根离子。阴极处的原位升高的pH值提供了CaP将变得高度过饱和的局部环境。因此,CaP的均匀和不均匀成核发生在阴极表面附近和阴极表面。由于局部pH高,P的去除行为对本体溶液的pH值不敏感,因此,在三种研究的pH分别为4.0(56.1%),8.2(57.4%)和10.0(48.4)的本体溶液中均观察到了有效的P去除。反应时间24小时后)。尽管脱磷效率通常不受本体溶液pH的影响,但通过结构表征证实,收集在阴极上的CaP固体的化学物理性质仍与本体溶液pH有关。较高的初始溶液pH值会促进形成具有较高Ca / P摩尔比的更多结晶产物。Ca / P摩尔比从1.30(pH 4.0)增加到1.38(pH 8.2),然后进一步增加到1.55(pH 10.0)。CaP沉淀物的形成是典型的结晶过程,在初始阶段形成无定形相,然后转变为最稳定的结晶相羟基磷灰石,这是由Ca / P摩尔比从1.38(第1天)提高至理论上为1.76(第11天)并由针状晶体形成。最后,我们证明了该系统对实际废水的效率。这与电化学方法可以在低总体pH值下工作而无需添加化学药品以及不需要分离过程的事实一起,突出了电化学方法在P去除和回收中的潜在应用。










































 京公网安备 11010802027423号
京公网安备 11010802027423号