当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Characterization of the Functional Variance in MbtH-like Protein Interactions with a Nonribosomal Peptide Synthetase
Biochemistry ( IF 2.9 ) Pub Date : 2017-09-20 00:00:00 , DOI: 10.1021/acs.biochem.7b00517 Rebecca A. Schomer 1 , Michael G. Thomas 1
Biochemistry ( IF 2.9 ) Pub Date : 2017-09-20 00:00:00 , DOI: 10.1021/acs.biochem.7b00517 Rebecca A. Schomer 1 , Michael G. Thomas 1
Affiliation
|
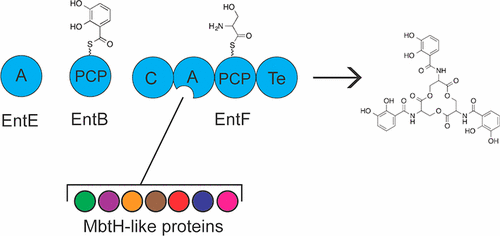
Many nonribosomal peptide synthetases (NRPSs) require MbtH-like proteins (MLPs) for solubility or for activation of amino acid substrate by the adenylation domain. MLPs are capable of functional crosstalk with noncognate NRPSs at varying levels. Using enterobactin biosynthesis in Escherichia coli as a model MLP-dependent NRPS system, we use in vivo and in vitro techniques to characterize how seven noncognate MLPs influence the function of the enterobactin NRPS EntF when the cognate MLP, YbdZ, is absent. Using a series of in vitro assays to analyze EntF solubility, adenylation, aminoacylation, and in vitro enterobactin production, we show that interactions between MLPs and NRPSs are multifaceted and more complex than previously appreciated. We separate MLP influence on solubility and function in a manner that shows altered solubility is not indicative of a functional MLP/NRPS pair. Although much of the functional variation among these noncognates can be explained by differences in EntF affinity for an MLP or the extent an MLP alters EntF l-Ser affinity, we demonstrate that MLPs can have a broader impact beyond solubility and adenylation. First, we show that a noncognate MLP can affect formation of l-Ser-S-EntF. Second, under in vitro conditions saturating for substrate and MLP, enterobactin production remains compromised in the absence of an appropriate MLP partner. These data suggest that we expand our investigations into how the MLPs influence NRPS enzymology. A more detailed understanding of these influences will be essential for downstream engineering of hybrid NRPS systems.
中文翻译:

与非核糖体肽合成酶的MbtH样蛋白相互作用中的功能差异的表征。
许多非核糖体肽合成酶(NRPS)需要MbtH样蛋白(MLP)才能溶解或通过腺苷酸化域激活氨基酸底物。MLP能够与不同级别的非同源NRPS进行功能串扰。使用大肠杆菌中的肠杆菌素生物合成作为模型MLP依赖性NRPS系统,我们使用体内和体外技术表征了当缺少关联MLP YbdZ时七个非关联MLP如何影响肠杆菌素NRPS EntF的功能。使用一系列体外分析来分析EntF溶解度,腺苷酸化,氨基酰化和体外肠杆菌素的生产,我们证明MLP和NRPS之间的相互作用是多方面的,并且比以前理解的更为复杂。我们以显示溶解度改变并不表示功能性MLP / NRPS对的方式分离MLP对溶解度和功能的影响。尽管这些不可识别物中的许多功能变异可以通过对MLP的EntF亲和力的差异或MLP改变EntF 1- Ser亲和力的程度来解释,但我们证明了MLP可以具有除溶解度和腺苷酸化以外的更广泛影响。首先,我们表明非同源的MLP可以影响1- Ser - SS - EntF的形成。二,体外在底物和MLP饱和的条件下,在缺乏合适的MLP配偶的情况下肠杆菌素的生产仍然受到损害。这些数据表明,我们将研究范围扩展到MLP如何影响NRPS酶学。对这些影响的更详细的了解对于混合NRPS系统的下游工程必不可少。
更新日期:2017-09-20
中文翻译:

与非核糖体肽合成酶的MbtH样蛋白相互作用中的功能差异的表征。
许多非核糖体肽合成酶(NRPS)需要MbtH样蛋白(MLP)才能溶解或通过腺苷酸化域激活氨基酸底物。MLP能够与不同级别的非同源NRPS进行功能串扰。使用大肠杆菌中的肠杆菌素生物合成作为模型MLP依赖性NRPS系统,我们使用体内和体外技术表征了当缺少关联MLP YbdZ时七个非关联MLP如何影响肠杆菌素NRPS EntF的功能。使用一系列体外分析来分析EntF溶解度,腺苷酸化,氨基酰化和体外肠杆菌素的生产,我们证明MLP和NRPS之间的相互作用是多方面的,并且比以前理解的更为复杂。我们以显示溶解度改变并不表示功能性MLP / NRPS对的方式分离MLP对溶解度和功能的影响。尽管这些不可识别物中的许多功能变异可以通过对MLP的EntF亲和力的差异或MLP改变EntF 1- Ser亲和力的程度来解释,但我们证明了MLP可以具有除溶解度和腺苷酸化以外的更广泛影响。首先,我们表明非同源的MLP可以影响1- Ser - SS - EntF的形成。二,体外在底物和MLP饱和的条件下,在缺乏合适的MLP配偶的情况下肠杆菌素的生产仍然受到损害。这些数据表明,我们将研究范围扩展到MLP如何影响NRPS酶学。对这些影响的更详细的了解对于混合NRPS系统的下游工程必不可少。











































 京公网安备 11010802027423号
京公网安备 11010802027423号