当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantioselective Synthesis of 1-Aryl-Substituted Tetrahydroisoquinolines Employing Imine Reductase
ACS Catalysis ( IF 11.3 ) Pub Date : 2017-09-20 00:00:00 , DOI: 10.1021/acscatal.7b02628 Jinmei Zhu 1 , Hongqun Tan 1 , Lu Yang 1 , Zheng Dai 1 , Lu Zhu 1 , Hongmin Ma 1 , Zixin Deng 1 , Zhenhua Tian 2 , Xudong Qu 1, 3
ACS Catalysis ( IF 11.3 ) Pub Date : 2017-09-20 00:00:00 , DOI: 10.1021/acscatal.7b02628 Jinmei Zhu 1 , Hongqun Tan 1 , Lu Yang 1 , Zheng Dai 1 , Lu Zhu 1 , Hongmin Ma 1 , Zixin Deng 1 , Zhenhua Tian 2 , Xudong Qu 1, 3
Affiliation
|
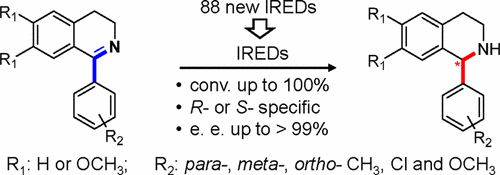
Tetrahydroisoquinolines (THIQs) with a C1-aryl-substituted groups are common in many natural and synthetic compounds of biological importance. Currently, their enantioselective synthesis are primarily reliant on chemical catalysis. Enzymatic synthesis using imine reductase is very attractive, because of the cost-effectiveness, high catalytic efficiency, and enantioselectivity. However, the steric hindrance of the 1-aryl substituents make this conversion very challenging, and current successful examples are mostly restricted to the simple alkyl-THIQs. In this report, through extensive evaluation of a large collection of IREDs (including 88 enzymes), we successfully identified a panel of steric-hindrance tolerated IREDs. These enzymes are able to convert meta- and para-substituted chloro-, methyl-, and methoxyl-benzyl dihydroisoquinolines (DHIQs) into corresponding R- or S- THIQs with very high enantioselectivity and conversion. Among them, the two most hindrance-tolerated enzymes (with different stereospecificity) are also able to convert ortho-substituted chloro-, methyl-, and methoxyl-benzyl DHIQs and dimethoxyl 1-chlorobenzyl-DHIQs with good enantiometric excess. Furthermore, using in silico modeling, a highly conserved tryptophan residue (W191) was identified to be critical for substrate accommodation in the binding cavity of the S-selective IRED (IR45). Replacing W191 with alanine can dramatically increase the catalytic performance by decreasing the Km value by 2 orders of magnitude. Our results provide an effective route to synthesize these important classes of THIQs. Moreover, the disclosed sequences and substrate binding model set a solid basis to generate more-efficient and broad-selective enzymes via protein engineering.
中文翻译:

使用亚胺还原酶的1-芳基取代的四氢异喹啉的对映选择性合成
具有C1-芳基取代基的四氢异喹啉(THIQs)在许多具有生物学重要性的天然和合成化合物中都很常见。目前,它们的对映选择性合成主要依赖于化学催化。由于成本效益,高催化效率和对映选择性,使用亚胺还原酶的酶促合成非常有吸引力。然而,1-芳基取代基的空间位阻使得这种转化非常具有挑战性,并且当前成功的实例大部分限于简单的烷基-THIQ。在本报告中,通过对大量IRED(包括88种酶)的广泛评估,我们成功鉴定出一组耐受空间位阻的IRED。这些酶能够转化间位和对位-取代的氯-,甲基-和甲氧基-苄基二氢异喹啉(DHIQs)转化为相应的R-或S- THIQs,具有很高的对映选择性和转化率。在它们当中,两种对障碍的耐受性最高的酶(具有不同的立体特异性)也能够以对映体过量良好的方式转化邻位取代的氯,甲基和甲氧基-苄基DHIQ和二甲氧基1-氯苄基-DHIQ。此外,使用计算机模拟,发现高度保守的色氨酸残基(W191)对于S选择性IRED(IR45)的结合腔中的底物调节至关重要。用丙氨酸代替W191可以通过降低K来显着提高催化性能m值增加2个数量级。我们的结果为综合这些重要的THIQ类提供了有效的途径。而且,所公开的序列和底物结合模型为通过蛋白质工程产生更有效和广泛选择的酶奠定了坚实的基础。
更新日期:2017-09-20
中文翻译:

使用亚胺还原酶的1-芳基取代的四氢异喹啉的对映选择性合成
具有C1-芳基取代基的四氢异喹啉(THIQs)在许多具有生物学重要性的天然和合成化合物中都很常见。目前,它们的对映选择性合成主要依赖于化学催化。由于成本效益,高催化效率和对映选择性,使用亚胺还原酶的酶促合成非常有吸引力。然而,1-芳基取代基的空间位阻使得这种转化非常具有挑战性,并且当前成功的实例大部分限于简单的烷基-THIQ。在本报告中,通过对大量IRED(包括88种酶)的广泛评估,我们成功鉴定出一组耐受空间位阻的IRED。这些酶能够转化间位和对位-取代的氯-,甲基-和甲氧基-苄基二氢异喹啉(DHIQs)转化为相应的R-或S- THIQs,具有很高的对映选择性和转化率。在它们当中,两种对障碍的耐受性最高的酶(具有不同的立体特异性)也能够以对映体过量良好的方式转化邻位取代的氯,甲基和甲氧基-苄基DHIQ和二甲氧基1-氯苄基-DHIQ。此外,使用计算机模拟,发现高度保守的色氨酸残基(W191)对于S选择性IRED(IR45)的结合腔中的底物调节至关重要。用丙氨酸代替W191可以通过降低K来显着提高催化性能m值增加2个数量级。我们的结果为综合这些重要的THIQ类提供了有效的途径。而且,所公开的序列和底物结合模型为通过蛋白质工程产生更有效和广泛选择的酶奠定了坚实的基础。











































 京公网安备 11010802027423号
京公网安备 11010802027423号