当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Direct Organocatalytic Multicomponent Synthesis of Enantiopure γ-Butyrolactones via Tandem Knoevenagel-Michael-Lactonization Sequence
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2017-09-20 06:25:44 , DOI: 10.1002/adsc.201701084 Tushar M. Khopade 1 , Amol D. Sonawane 1 , Jyotsna S. Arora 1 , Ramakrishna G. Bhat 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2017-09-20 06:25:44 , DOI: 10.1002/adsc.201701084 Tushar M. Khopade 1 , Amol D. Sonawane 1 , Jyotsna S. Arora 1 , Ramakrishna G. Bhat 1
Affiliation
|
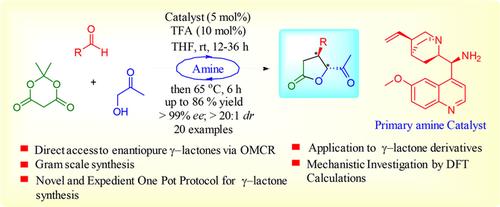
An expedient and straightforward protocol is developed for the synthesis of highly enantiopure synthesis of γ-butyrolactones. For the first time, one pot enantioselective organocatalytic multicomponent reaction (OMCR) is explored to construct functionalized butyrolactones without the use of pre-functionalized substrates and expensive transition metals. The protocol is proved to be reproducible on a gram scale. Density functional theory (DFT) calculations strongly support the mechanism and were in close agreement with the observed high stereoselectivity.
中文翻译:

通过串联Knoevenagel-Michael-内酯化序列直接有机催化多组分合成对映体纯γ-丁内酯
为合成高度对映纯的γ-丁内酯开发了一种简便而直接的方案。首次探索了一个锅对映选择性有机催化多组分反应(OMCR),无需使用预功能化的底物和昂贵的过渡金属即可构建功能化的丁内酯。该协议被证明在克规模上是可重现的。密度泛函理论(DFT)的计算有力地支持了这一机理,并与观察到的高立体选择性高度吻合。
更新日期:2017-09-20
中文翻译:

通过串联Knoevenagel-Michael-内酯化序列直接有机催化多组分合成对映体纯γ-丁内酯
为合成高度对映纯的γ-丁内酯开发了一种简便而直接的方案。首次探索了一个锅对映选择性有机催化多组分反应(OMCR),无需使用预功能化的底物和昂贵的过渡金属即可构建功能化的丁内酯。该协议被证明在克规模上是可重现的。密度泛函理论(DFT)的计算有力地支持了这一机理,并与观察到的高立体选择性高度吻合。











































 京公网安备 11010802027423号
京公网安备 11010802027423号