当前位置:
X-MOL 学术
›
J. Mater. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Formation of toroids by self-assembly of an α–α corner mimetic: supramolecular cyclization
Journal of Materials Chemistry B ( IF 6.1 ) Pub Date : 2017-08-22 00:00:00 , DOI: 10.1039/c7tb01711a Debasish Podder 1, 2, 3, 4 , Santu Bera 1, 2, 3, 4 , Mintu Debnath 1, 2, 3, 4 , Tanmay Das 1, 2, 3, 4 , Debasish Haldar 1, 2, 3, 4
Journal of Materials Chemistry B ( IF 6.1 ) Pub Date : 2017-08-22 00:00:00 , DOI: 10.1039/c7tb01711a Debasish Podder 1, 2, 3, 4 , Santu Bera 1, 2, 3, 4 , Mintu Debnath 1, 2, 3, 4 , Tanmay Das 1, 2, 3, 4 , Debasish Haldar 1, 2, 3, 4
Affiliation
|
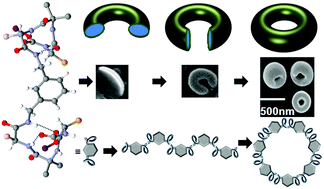
An α–α corner mimetic self-assembles to form a rod-like supramolecular structure which bends and closes end-to-end like a cyclization reaction to form uniform toroids. Each peptide fragment containing L-leucine, α-aminoisobutyric acid (Aib) and L-tyrosine forms rigid 310 helical structures stabilized by multiple intramolecular N–H⋯O hydrogen bonds. Two 310 helices are connected by the spacer 3-aminomethyl-benzylamine and maintain an angular distance of 120° and therefore mimic the α–α corner motif of a protein super secondary structure. The individual α–α corner subunits are themselves regularly interlinked through multiple water mediated intermolecular hydrogen-bonding interactions to form the rod-like supramolecular structure and toroids. The formation of the supramolecular structure has been proven with X-ray crystallography and other spectroscopic techniques. The cyclization of the supramolecular structure and toroid formation were studied by optical microscope, AFM and FE-SEM experiments. Despite other assignments such as exfoliation of graphene from graphite, the compound exhibits significant memory to finally produce the toroids.
中文翻译:

通过α-α角模拟物的自组装形成环面:超分子环化
一个α-α角模拟物自组装形成杆状超分子结构,该结构像环化反应一样端对端弯曲和闭合以形成均匀的环面。每个包含L-亮氨酸,α-氨基异丁酸(Aib)和L-酪氨酸的肽片段均形成由多个分子内N–H⋯O氢键稳定的3-10个刚性螺旋结构。两个3 10螺旋通过间隔基3-氨基甲基-苄胺连接,并保持120°的角距离,因此模仿了蛋白质超级二级结构的α-α角基序。各个α–α角亚基本身通过多种水介导的分子间氢键相互作用而定期相互连接,从而形成棒状超分子结构和环型环。X射线晶体学和其他光谱技术已证明了超分子结构的形成。通过光学显微镜,原子力显微镜和FE-SEM实验研究了超分子结构的环化和环型的形成。尽管有其他任务,例如将石墨烯从石墨上剥落,该化合物仍具有显着的记忆力,可最终生产出超环面材料。
更新日期:2017-09-20
中文翻译:

通过α-α角模拟物的自组装形成环面:超分子环化
一个α-α角模拟物自组装形成杆状超分子结构,该结构像环化反应一样端对端弯曲和闭合以形成均匀的环面。每个包含L-亮氨酸,α-氨基异丁酸(Aib)和L-酪氨酸的肽片段均形成由多个分子内N–H⋯O氢键稳定的3-10个刚性螺旋结构。两个3 10螺旋通过间隔基3-氨基甲基-苄胺连接,并保持120°的角距离,因此模仿了蛋白质超级二级结构的α-α角基序。各个α–α角亚基本身通过多种水介导的分子间氢键相互作用而定期相互连接,从而形成棒状超分子结构和环型环。X射线晶体学和其他光谱技术已证明了超分子结构的形成。通过光学显微镜,原子力显微镜和FE-SEM实验研究了超分子结构的环化和环型的形成。尽管有其他任务,例如将石墨烯从石墨上剥落,该化合物仍具有显着的记忆力,可最终生产出超环面材料。











































 京公网安备 11010802027423号
京公网安备 11010802027423号