当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Base-mediated diastereoselective [4 + 3] annulation of in situ generated ortho-quinone methides with C,N-cyclic azomethine imines
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2017-08-29 00:00:00 , DOI: 10.1039/c7ob01783a Jianfeng Xu 1, 2, 3, 4 , Shiru Yuan 1, 2, 3, 4 , Jingyi Peng 1, 2, 3, 4 , Maozhong Miao 1, 2, 3, 4 , Zhengkai Chen 1, 2, 3, 4 , Hongjun Ren 1, 2, 3, 4
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2017-08-29 00:00:00 , DOI: 10.1039/c7ob01783a Jianfeng Xu 1, 2, 3, 4 , Shiru Yuan 1, 2, 3, 4 , Jingyi Peng 1, 2, 3, 4 , Maozhong Miao 1, 2, 3, 4 , Zhengkai Chen 1, 2, 3, 4 , Hongjun Ren 1, 2, 3, 4
Affiliation
|
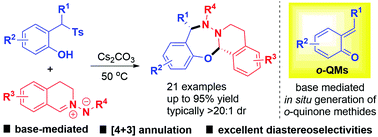
An efficient [4 + 3] annulation of 2-(1-tosylalkyl)phenols with C,N-cyclic azomethine imines via in situ generation of ortho-quinone methides (o-QMs) under mild basic reaction conditions is disclosed, furnishing biologically interesting seven-membered heterocyclic compounds with moderate to good yields and excellent diastereoselectivities. A gram-scale reaction is performed to demonstrate the potential in industrial application and two transition states are proposed to rationalize the outstanding diastereoselectivity.
中文翻译:

碱介导的原位产生的邻醌甲基化物与C,N环偶氮甲亚胺的非对映选择性[4 + 3]环化
公开了在温和的碱性反应条件下通过原位生成邻醌甲基化物(o -QMs)使2-(1-甲苯基烷基)苯酚与C,N-环偶氮甲亚胺有效的[4 + 3]环化反应,具有中等至良好收率和出色的非对映选择性的七元杂环化合物。进行了克级反应以证明其在工业应用中的潜力,并提出了两种过渡态以合理化出色的非对映选择性。
更新日期:2017-09-20
中文翻译:

碱介导的原位产生的邻醌甲基化物与C,N环偶氮甲亚胺的非对映选择性[4 + 3]环化
公开了在温和的碱性反应条件下通过原位生成邻醌甲基化物(o -QMs)使2-(1-甲苯基烷基)苯酚与C,N-环偶氮甲亚胺有效的[4 + 3]环化反应,具有中等至良好收率和出色的非对映选择性的七元杂环化合物。进行了克级反应以证明其在工业应用中的潜力,并提出了两种过渡态以合理化出色的非对映选择性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号