当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Oxidative C–H functionalization of N-carbamoyl 1,2-dihydroquinolines
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2017-08-25 00:00:00 , DOI: 10.1039/c7ob01793f Ziqiang Liu 1, 2, 3, 4, 5 , Lei Chen 3, 6, 7, 8 , Jing Li 3, 9, 10, 11 , Ke Liu 3, 9, 10, 11 , Jiaqi Zhao 3, 9, 10, 11 , Mengmeng Xu 3, 9, 10, 11 , Lei Feng 3, 9, 10, 11 , Ren-zhong Wan 3, 6, 7, 8 , Wei Li 3, 4, 5, 12, 13 , Lei Liu 1, 2, 3, 9, 10
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2017-08-25 00:00:00 , DOI: 10.1039/c7ob01793f Ziqiang Liu 1, 2, 3, 4, 5 , Lei Chen 3, 6, 7, 8 , Jing Li 3, 9, 10, 11 , Ke Liu 3, 9, 10, 11 , Jiaqi Zhao 3, 9, 10, 11 , Mengmeng Xu 3, 9, 10, 11 , Lei Feng 3, 9, 10, 11 , Ren-zhong Wan 3, 6, 7, 8 , Wei Li 3, 4, 5, 12, 13 , Lei Liu 1, 2, 3, 9, 10
Affiliation
|
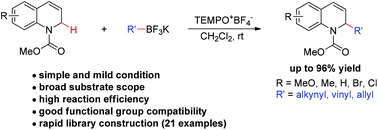
A modular and efficient method for the synthesis of α-substituted 1,2-dihydroquinolines is described. Under mild metal-free conditions, readily available N-carbamoyl 1,2-dihydroquinolines undergo oxidative C–H alkynylation, alkenylation, and allylation with a range of potassium trifluoroborates using TEMPO oxoammonium salt as an oxidant.
中文翻译:

N-氨基甲酰基1,2-二氢喹啉的氧化C–H功能化
描述了用于合成α-取代的1,2-二氢喹啉的模块化且有效的方法。在温和的不含金属的条件下,使用TEMPO氧铵盐作为氧化剂,容易获得的N-氨基甲酰基1,2-二氢喹啉会与一系列三氟硼酸钾进行氧化性C–H炔基化,烯基化和烯丙基化。
更新日期:2017-09-20
中文翻译:

N-氨基甲酰基1,2-二氢喹啉的氧化C–H功能化
描述了用于合成α-取代的1,2-二氢喹啉的模块化且有效的方法。在温和的不含金属的条件下,使用TEMPO氧铵盐作为氧化剂,容易获得的N-氨基甲酰基1,2-二氢喹啉会与一系列三氟硼酸钾进行氧化性C–H炔基化,烯基化和烯丙基化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号