当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stereodivergent Mannich reaction of bis(trimethylsilyl)ketene acetals with N-tert-butanesulfinyl imines by Lewis acid or Lewis base activation, a one-pot protocol to obtain chiral β-amino acids
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2017-08-24 00:00:00 , DOI: 10.1039/c7ob01853c Margarita Cantú-Reyes 1, 2, 3, 4, 5 , Isabel Alvarado-Beltrán 1, 2, 3, 4, 5 , Ricardo Ballinas-Indilí 1, 2, 3, 4, 5 , Cecilio Álvarez-Toledano 1, 2, 3, 4, 5 , Marcos Hernández-Rodríguez 1, 2, 3, 4, 5
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2017-08-24 00:00:00 , DOI: 10.1039/c7ob01853c Margarita Cantú-Reyes 1, 2, 3, 4, 5 , Isabel Alvarado-Beltrán 1, 2, 3, 4, 5 , Ricardo Ballinas-Indilí 1, 2, 3, 4, 5 , Cecilio Álvarez-Toledano 1, 2, 3, 4, 5 , Marcos Hernández-Rodríguez 1, 2, 3, 4, 5
Affiliation
|
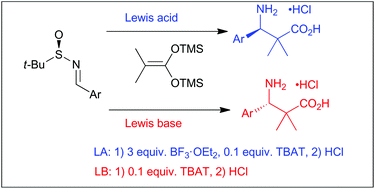
We report a one-pot synthesis of chiral β2,2,3-amino acids by the Mannich addition of bistrimethylsilyl ketene acetals to N-tert-butanesulfinyl imines followed by the removal of the chiral auxiliary. The synthesis and isolation of pure β-amino acid hydrochlorides were conducted under mild conditions, without strong bases and this method is operationally simple. The stereoselective reaction was promoted by two different activation methods that lead to different stereoisomers: (1) Lewis Acid (LA) catalysis with boron trifluoride diethyl etherate and (2) Lewis Base (LB) catalysis with tetrabutylammonium difluorotriphenylsilicate. The reaction presented good diastereoselectivity with LB activation and moderate to good dr with LA catalysis. The exceptions in both protocols were imines with electron donating groups in the aromatic ring.
中文翻译:

双(三甲基甲硅烷基)乙烯酮缩醛与N-叔丁亚磺酰基亚胺通过路易斯酸或路易斯碱活化的立体发散曼尼希反应,通过一锅法获得手性β-氨基酸
我们报告了一锅合成的手性β2,2,3-氨基酸由曼尼希的双三甲基甲硅烷基乙烯酮缩醛缩合至N-叔胺-丁烷亚磺酰基亚胺,然后除去手性助剂。纯β-氨基酸盐酸盐的合成和分离是在温和的条件下进行的,没有强碱,该方法操作简单。通过两种导致不同立体异构体的活化方法促进了立体选择性反应:(1)用三氟化硼二乙基醚化物的路易斯酸(LA)催化和(2)用二丁基三氟硅酸四铵铵的路易斯碱(LB)催化。该反应在LB活化下表现出良好的非对映选择性,在LA催化下表现出中等至良好的dr。两种方案中的例外情况都是亚胺在芳环中带有给电子基团。
更新日期:2017-09-20
中文翻译:

双(三甲基甲硅烷基)乙烯酮缩醛与N-叔丁亚磺酰基亚胺通过路易斯酸或路易斯碱活化的立体发散曼尼希反应,通过一锅法获得手性β-氨基酸
我们报告了一锅合成的手性β2,2,3-氨基酸由曼尼希的双三甲基甲硅烷基乙烯酮缩醛缩合至N-叔胺-丁烷亚磺酰基亚胺,然后除去手性助剂。纯β-氨基酸盐酸盐的合成和分离是在温和的条件下进行的,没有强碱,该方法操作简单。通过两种导致不同立体异构体的活化方法促进了立体选择性反应:(1)用三氟化硼二乙基醚化物的路易斯酸(LA)催化和(2)用二丁基三氟硅酸四铵铵的路易斯碱(LB)催化。该反应在LB活化下表现出良好的非对映选择性,在LA催化下表现出中等至良好的dr。两种方案中的例外情况都是亚胺在芳环中带有给电子基团。











































 京公网安备 11010802027423号
京公网安备 11010802027423号