当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Intramolecular hydrogen bonding in conformationally semi-rigid α-acylmethane derivatives: a theoretical NMR study
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2017-08-18 00:00:00 , DOI: 10.1039/c7ob01834g Antonio J. Mota 1, 2, 3, 4 , Jürgen Neuhold 5, 6, 7, 8 , Martina Drescher 5, 7, 8, 9 , Sébastien Lemouzy 5, 7, 8, 9 , Leticia González 5, 6, 7, 8 , Nuno Maulide 5, 7, 8, 9
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2017-08-18 00:00:00 , DOI: 10.1039/c7ob01834g Antonio J. Mota 1, 2, 3, 4 , Jürgen Neuhold 5, 6, 7, 8 , Martina Drescher 5, 7, 8, 9 , Sébastien Lemouzy 5, 7, 8, 9 , Leticia González 5, 6, 7, 8 , Nuno Maulide 5, 7, 8, 9
Affiliation
|
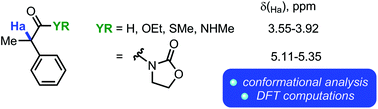
Conformational mobility is a core property of organic compounds, and conformational analysis has become a pervasive tool for synthetic design. In this work, we present experimental and computational (employing Density Functional Theory) evidence for unusual intramolecular hydrogen bonding interactions in a series of α-acylmethane derivatives, as well as a discussion of the consequences thereof for their NMR spectroscopic properties.
中文翻译:

构象半刚性α-酰基甲烷衍生物中的分子内氢键:理论NMR研究
构象迁移是有机化合物的核心特性,构象分析已成为合成设计的普遍工具。在这项工作中,我们提供了一系列α-酰基甲烷衍生物中异常分子内氢键相互作用的实验和计算证据(采用密度泛函理论),并讨论了其对NMR光谱性质的影响。
更新日期:2017-09-20
中文翻译:

构象半刚性α-酰基甲烷衍生物中的分子内氢键:理论NMR研究
构象迁移是有机化合物的核心特性,构象分析已成为合成设计的普遍工具。在这项工作中,我们提供了一系列α-酰基甲烷衍生物中异常分子内氢键相互作用的实验和计算证据(采用密度泛函理论),并讨论了其对NMR光谱性质的影响。










































 京公网安备 11010802027423号
京公网安备 11010802027423号