当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Novel pyrrolobenzodiazepine and pyrroloquinazoline scaffolds synthesized by a simple and highly selective Ugi/cyclization sequence
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2017-08-18 00:00:00 , DOI: 10.1039/c7ob01807j Pablo Pertejo 1, 2, 3, 4, 5 , Pablo Peña-Calleja 1, 2, 3, 4, 5 , Israel Carreira-Barral 1, 2, 3, 4, 5 , Roberto Quesada 1, 2, 3, 4, 5 , Nicolás Alejandro Cordero 2, 3, 4, 5, 6 , Francisco Javier Rodríguez 1, 3, 4, 5, 7 , María García-Valverde 1, 2, 3, 4, 5
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2017-08-18 00:00:00 , DOI: 10.1039/c7ob01807j Pablo Pertejo 1, 2, 3, 4, 5 , Pablo Peña-Calleja 1, 2, 3, 4, 5 , Israel Carreira-Barral 1, 2, 3, 4, 5 , Roberto Quesada 1, 2, 3, 4, 5 , Nicolás Alejandro Cordero 2, 3, 4, 5, 6 , Francisco Javier Rodríguez 1, 3, 4, 5, 7 , María García-Valverde 1, 2, 3, 4, 5
Affiliation
|
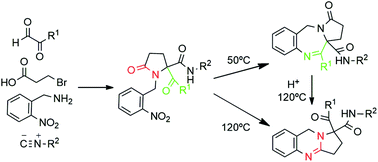
Pyrrolo[2,1-c][1,4]benzodiazepines (PBDs) and other benzo-fused N-heterocycles constitute privileged structures found in numerous bioactive compounds. Thus, developing simple and selective syntheses to furnish these derivatives from easily accessible starting materials is an important and challenging goal. In this work, novel pyrrolobenzodiazepine and pyrroloquinazoline derivatives have been synthesized following a common two step synthetic strategy. This strategy involves a one-pot Ugi/cyclization sequence followed by a reduction with spontaneous thermocontrolled cyclization. The control of the temperature in this second step allows fully selective access to either pyrrolo[2,1-c][1,4]benzodiazepine-3-ones 6 or pyrrolo[2,1-b]quinazolines 7. Density functional theory (DFT) calculations have been carried out to rationalize this reactivity, identifying the kinetic and thermodynamic reaction products and offering insights into the cyclization pathways. These synthetic methodologies show the versatility of the Ugi reaction as a tool in the synthesis of heterocyclic compounds with a pseudopeptidic skeleton.
中文翻译:

通过简单和高度选择性的Ugi /环化序列合成的新型吡咯并苯并二氮杂卓和吡咯并喹唑啉支架
吡咯并[ 2,1- c ] [1,4]苯并二氮杂卓(PBD)和其他苯并稠合的N-杂环构成在许多生物活性化合物中发现的特权结构。因此,开发简单且选择性的合成物以从容易获得的起始原料中提供这些衍生物是重要且具有挑战性的目标。在这项工作中,按照常见的两步合成策略合成了新颖的吡咯并苯并二氮杂卓和吡咯并喹唑啉衍生物。此策略涉及一锅Ugi /环化序列,然后通过自发的温度控制环化进行还原。在第二步中控制温度允许完全选择性地获得吡咯并[2,1- c ] [1,4]苯并二氮杂-3-酮6或吡咯并[2,1-b ]喹唑啉7。进行了密度泛函理论(DFT)计算以合理化这种反应性,确定了动力学和热力学反应产物,并提供了有关环化途径的见解。这些合成方法论表明,Ugi反应作为具有假肽骨架的杂环化合物的合成工具具有多功能性。
更新日期:2017-09-20
中文翻译:

通过简单和高度选择性的Ugi /环化序列合成的新型吡咯并苯并二氮杂卓和吡咯并喹唑啉支架
吡咯并[ 2,1- c ] [1,4]苯并二氮杂卓(PBD)和其他苯并稠合的N-杂环构成在许多生物活性化合物中发现的特权结构。因此,开发简单且选择性的合成物以从容易获得的起始原料中提供这些衍生物是重要且具有挑战性的目标。在这项工作中,按照常见的两步合成策略合成了新颖的吡咯并苯并二氮杂卓和吡咯并喹唑啉衍生物。此策略涉及一锅Ugi /环化序列,然后通过自发的温度控制环化进行还原。在第二步中控制温度允许完全选择性地获得吡咯并[2,1- c ] [1,4]苯并二氮杂-3-酮6或吡咯并[2,1-b ]喹唑啉7。进行了密度泛函理论(DFT)计算以合理化这种反应性,确定了动力学和热力学反应产物,并提供了有关环化途径的见解。这些合成方法论表明,Ugi反应作为具有假肽骨架的杂环化合物的合成工具具有多功能性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号