当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Formation of hydrazones and stabilized boron–nitrogen heterocycles in aqueous solution from carbohydrazides and ortho-formylphenylboronic acids
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2017-08-08 00:00:00 , DOI: 10.1039/c7ob01708a Han Gu 1, 2, 3, 4, 5 , Tak Ian Chio 1, 2, 3, 4, 5 , Zhen Lei 1, 2, 3, 4, 5 , Richard J. Staples 5, 6, 7, 8 , Jennifer S. Hirschi 1, 2, 3, 4, 5 , Susan Bane 1, 2, 3, 4, 5
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2017-08-08 00:00:00 , DOI: 10.1039/c7ob01708a Han Gu 1, 2, 3, 4, 5 , Tak Ian Chio 1, 2, 3, 4, 5 , Zhen Lei 1, 2, 3, 4, 5 , Richard J. Staples 5, 6, 7, 8 , Jennifer S. Hirschi 1, 2, 3, 4, 5 , Susan Bane 1, 2, 3, 4, 5
Affiliation
|
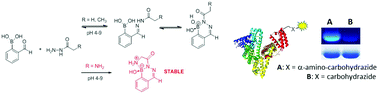
A recent addition to the suite of fast bioorthogonal reactions combines hydrazines and hydroxylamines with ortho-carbonyl substituted phenylboronic acids. Carbohydrazides are easily incorporated into biomolecules, making them appealing substrates in these reactions. Here we show that simple alkyl carbohydrazides form a single product with ortho-formylphenylboronic acid in an organic solvent and in the solid state. The solution structures of the products formed from the carbohydrazides in buffered aqueous solution, however, are markedly different from those identified in the organic solvent and solid state. The reactants form a mixture of hydrazone and heterocyclic products, the relative composition of which varies with pH. The observed reversibility of bioconjugates using carbohydrazide can thus be explained by the reversibility of the boron–nitrogen bond in the heterocycle. In contrast, the inclusion of an α-amine into the carbohydrazide substrate yields a single product in which both nitrogens are bonded to boron. These tricyclic structures are the same in organic solvent, solid state and aqueous solution from pH 4 to pH 9. Bioconjugates formed with α-amino carbohydrazides are stable to SDS-PAGE, while those formed with simple alkyl carbohydrazides are not. We propose that the inclusion of an intramolecular stabilizing ligand into a carbohydrazide substrate is a generally applicable principle that may be exploited to form boronic acid-based bioconjugates with a defined structure and resistance to hydrolysis.
中文翻译:

由碳酰肼和邻甲酰基苯基硼酸在水溶液中形成hydr和稳定的硼氮杂环
这套快速的生物正交反应的最新进展是将肼和羟胺与邻羰基取代的苯基硼酸结合在一起。碳酰肼很容易掺入生物分子中,使其成为这些反应中有吸引力的底物。在这里,我们证明简单的烷基碳酰肼与邻位形成单一产物-甲酰基苯基硼酸在有机溶剂中并呈固态。然而,由碳酰肼在缓冲水溶液中形成的溶液结构与在有机溶剂和固态下确定的结构明显不同。反应物形成of和杂环产物的混合物,其相对组成随pH而变化。因此,使用碳酰肼观察到的生物共轭物的可逆性可以通过杂环中硼-氮键的可逆性来解释。相反,将α-胺包含到碳酰肼底物中产生了单一产物,其中两个氮都结合到硼上。这些三环结构在有机溶剂,固态和从pH到pH 9的水溶液中是相同的。由α-氨基碳酰肼形成的生物结合物对SDS-PAGE稳定,而与简单的烷基碳酰肼形成的生物结合物则不稳定。我们建议将分子内稳定配体包含在碳酰肼底物中是普遍适用的原理,可以利用该原理来形成具有确定的结构和抗水解性的基于硼酸的生物缀合物。
更新日期:2017-09-20
中文翻译:

由碳酰肼和邻甲酰基苯基硼酸在水溶液中形成hydr和稳定的硼氮杂环
这套快速的生物正交反应的最新进展是将肼和羟胺与邻羰基取代的苯基硼酸结合在一起。碳酰肼很容易掺入生物分子中,使其成为这些反应中有吸引力的底物。在这里,我们证明简单的烷基碳酰肼与邻位形成单一产物-甲酰基苯基硼酸在有机溶剂中并呈固态。然而,由碳酰肼在缓冲水溶液中形成的溶液结构与在有机溶剂和固态下确定的结构明显不同。反应物形成of和杂环产物的混合物,其相对组成随pH而变化。因此,使用碳酰肼观察到的生物共轭物的可逆性可以通过杂环中硼-氮键的可逆性来解释。相反,将α-胺包含到碳酰肼底物中产生了单一产物,其中两个氮都结合到硼上。这些三环结构在有机溶剂,固态和从pH到pH 9的水溶液中是相同的。由α-氨基碳酰肼形成的生物结合物对SDS-PAGE稳定,而与简单的烷基碳酰肼形成的生物结合物则不稳定。我们建议将分子内稳定配体包含在碳酰肼底物中是普遍适用的原理,可以利用该原理来形成具有确定的结构和抗水解性的基于硼酸的生物缀合物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号