当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cu(II)-Mediated keto C(sp3)–H bond α-acyloxylation of N,N-dialkylamides with aromatic carboxylic acids
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2017-07-11 00:00:00 , DOI: 10.1039/c7ob01190c Wenjing Li 1, 2, 3, 4, 5 , Changzhen Yin 1, 2, 3, 4, 5 , Xiao Yang 1, 2, 3, 4, 5 , Hailong Liu 1, 2, 3, 4, 5 , Xueli Zheng 1, 2, 3, 4, 5 , Maolin Yuan 1, 2, 3, 4, 5 , Ruixiang Li 1, 2, 3, 4, 5 , Haiyan Fu 1, 2, 3, 4, 5 , Hua Chen 1, 2, 3, 4, 5
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2017-07-11 00:00:00 , DOI: 10.1039/c7ob01190c Wenjing Li 1, 2, 3, 4, 5 , Changzhen Yin 1, 2, 3, 4, 5 , Xiao Yang 1, 2, 3, 4, 5 , Hailong Liu 1, 2, 3, 4, 5 , Xueli Zheng 1, 2, 3, 4, 5 , Maolin Yuan 1, 2, 3, 4, 5 , Ruixiang Li 1, 2, 3, 4, 5 , Haiyan Fu 1, 2, 3, 4, 5 , Hua Chen 1, 2, 3, 4, 5
Affiliation
|
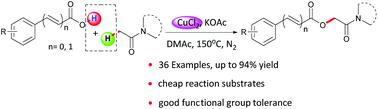
The selective oxidative coupling of aromatic carboxylic acids with the C(sp3)–H bond adjacent to the keto group of alkylamides has been developed by employing a low cost copper source. This provides an efficient approach for synthesis of O-benzoylglycolamides. The protocol displayed good functional group tolerance. A broad range of benzoic acids directly coupled with alkylamides to afford a variety of O-benzoylglycolamides in moderate to good yields. In addition, a reasonable radical mechanism was proposed based on EPR experiments.
中文翻译:

Cu(II)介导的N,N-二烷基酰胺与芳族羧酸的酮C(sp 3)-H键的α-酰氧基化
通过使用低成本的铜源,已开发出芳香羧酸与邻近烷基酰胺酮基的C(sp 3)-H键的选择性氧化偶联。这提供了合成O-苯甲酰基甘醇酰胺的有效方法。该协议显示出良好的功能组耐受性。大量的苯甲酸直接与烷基酰胺偶联,以中等到良好的产率提供了各种O-苯甲酰基甘醇酰胺。另外,在EPR实验的基础上,提出了一种合理的自由基机理。
更新日期:2017-09-20
中文翻译:

Cu(II)介导的N,N-二烷基酰胺与芳族羧酸的酮C(sp 3)-H键的α-酰氧基化
通过使用低成本的铜源,已开发出芳香羧酸与邻近烷基酰胺酮基的C(sp 3)-H键的选择性氧化偶联。这提供了合成O-苯甲酰基甘醇酰胺的有效方法。该协议显示出良好的功能组耐受性。大量的苯甲酸直接与烷基酰胺偶联,以中等到良好的产率提供了各种O-苯甲酰基甘醇酰胺。另外,在EPR实验的基础上,提出了一种合理的自由基机理。









































 京公网安备 11010802027423号
京公网安备 11010802027423号