当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Two-dimensional pattern formation in ionic liquids confined between graphene walls
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2017-09-11 00:00:00 , DOI: 10.1039/c7cp04649a Hadrián Montes-Campos 1, 2, 3, 4, 5 , José Manuel Otero-Mato 1, 2, 3, 4, 5 , Trinidad Méndez-Morales 1, 2, 3, 4, 5 , Oscar Cabeza 6, 7, 8, 9, 10 , Luis J. Gallego 1, 2, 3, 4, 5 , Alina Ciach 11, 12, 13, 14 , Luis M. Varela 1, 2, 3, 4, 5
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2017-09-11 00:00:00 , DOI: 10.1039/c7cp04649a Hadrián Montes-Campos 1, 2, 3, 4, 5 , José Manuel Otero-Mato 1, 2, 3, 4, 5 , Trinidad Méndez-Morales 1, 2, 3, 4, 5 , Oscar Cabeza 6, 7, 8, 9, 10 , Luis J. Gallego 1, 2, 3, 4, 5 , Alina Ciach 11, 12, 13, 14 , Luis M. Varela 1, 2, 3, 4, 5
Affiliation
|
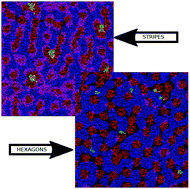
We perform molecular dynamics simulations of ionic liquids confined between graphene walls under a large variety of conditions (pure ionic liquids, mixtures with water and alcohols, mixtures with lithium salts and defective graphene walls). Our results show that the formation of striped and hexagonal patterns in the Stern layer can be considered as a general feature of ionic liquids at electrochemical interfaces, the transition between patterns being controlled by the net balance of charge in the innermost layer of adsorbed molecules. This explains previously reported experimental and computational results and, for the first time, why these pattern changes are triggered by any perturbation of the charge density at the innermost layer of the electric double layer (voltage and composition changes, and vacancies at the electrode walls, among others), which may help tuning electrode–ionic liquid interfaces. Using Monte Carlo simulations we show that such structures can be reproduced by a simple two-dimensional lattice model with only nearest-neighbour interactions, governed by highly screened ionic interactions and short-range and excluded volume interactions. We also show that the results of our simulations are consistent with those inferred from the Landau–Brazovskii theory of pattern formation in self-assembling systems. The presence of these patterns at the ionic liquid graphene–electrode interfaces may have a strong impact on the process of ionic transfer from the bulk mixtures to the electrodes, on the differential capacitance of the electrode–electrolyte double layer or on the rates of redox reactions at the electrodes, among other physicochemical properties, and is therefore an effect of great technological interest.
中文翻译:

限制在石墨烯壁之间的离子液体中的二维图案形成
我们在多种条件下(纯离子液体,与水和醇的混合物,与锂盐的混合物和有缺陷的石墨烯壁)对封闭在石墨烯壁之间的离子液体进行分子动力学模拟。我们的结果表明,在Stern层中形成条纹和六边形图案可被视为离子液体在电化学界面上的一般特征,图案之间的过渡受吸附分子最内层的净电荷平衡控制。这解释了先前报道的实验和计算结果,并且首次解释了为什么这些图案变化是由双电层最内层的电荷密度的任何扰动(电压和成分变化以及电极壁的空位)引起的,等等),这可能有助于调整电极-离子液体界面。使用蒙特卡洛模拟,我们表明可以通过仅具有最近邻相互作用的简单二维晶格模型来再现此类结构,该二维晶格模型受高度筛选的离子相互作用以及短程和排除体积的相互作用的支配。我们还表明,我们的模拟结果与从Landau-Brazovskii自组装系统中的图案形成理论推论得出的结果一致。这些图案在离子液体石墨烯-电极界面处的存在可能会对离子从本体混合物转移到电极的过程,电极-电解质双层的微分电容或氧化还原反应的速率产生重大影响在电极上,以及其他物理化学特性
更新日期:2017-09-20
中文翻译:

限制在石墨烯壁之间的离子液体中的二维图案形成
我们在多种条件下(纯离子液体,与水和醇的混合物,与锂盐的混合物和有缺陷的石墨烯壁)对封闭在石墨烯壁之间的离子液体进行分子动力学模拟。我们的结果表明,在Stern层中形成条纹和六边形图案可被视为离子液体在电化学界面上的一般特征,图案之间的过渡受吸附分子最内层的净电荷平衡控制。这解释了先前报道的实验和计算结果,并且首次解释了为什么这些图案变化是由双电层最内层的电荷密度的任何扰动(电压和成分变化以及电极壁的空位)引起的,等等),这可能有助于调整电极-离子液体界面。使用蒙特卡洛模拟,我们表明可以通过仅具有最近邻相互作用的简单二维晶格模型来再现此类结构,该二维晶格模型受高度筛选的离子相互作用以及短程和排除体积的相互作用的支配。我们还表明,我们的模拟结果与从Landau-Brazovskii自组装系统中的图案形成理论推论得出的结果一致。这些图案在离子液体石墨烯-电极界面处的存在可能会对离子从本体混合物转移到电极的过程,电极-电解质双层的微分电容或氧化还原反应的速率产生重大影响在电极上,以及其他物理化学特性











































 京公网安备 11010802027423号
京公网安备 11010802027423号