当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A highly enantioselective asymmetric Darzens reaction catalysed by proline based efficient organocatalysts for the synthesis of di- and tri-substituted epoxides
Chemical Communications ( IF 4.9 ) Pub Date : 2017-09-13 00:00:00 , DOI: 10.1039/c7cc06194c Veeramanoharan Ashokkumar 1, 2, 3, 4, 5 , Ayyanar Siva 1, 2, 3, 4, 5 , R. Ramaswamy Chidambaram 1, 2, 3, 4, 5
Chemical Communications ( IF 4.9 ) Pub Date : 2017-09-13 00:00:00 , DOI: 10.1039/c7cc06194c Veeramanoharan Ashokkumar 1, 2, 3, 4, 5 , Ayyanar Siva 1, 2, 3, 4, 5 , R. Ramaswamy Chidambaram 1, 2, 3, 4, 5
Affiliation
|
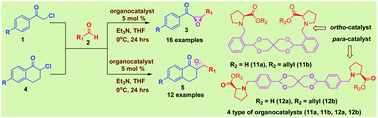
A new class of easily available and readily tunable proline based chiral organocatalysts was found to efficiently catalyse an unprecedented highly enantioselective asymmetric Darzens reaction of α-chloroketones and substituted α-chloroketones with various aldehydes, which directly produces optically active di- and tri-substituted chiral epoxides with higher product yields (up to 97%) and excellent ee's (up to 99%) under mild reaction conditions.
中文翻译:

脯氨酸基高效有机催化剂催化的高对映选择性不对称Darzens反应,用于合成二取代和三取代的环氧化物
已发现一类新的易于获得且易于调节的脯氨酸基手性有机催化剂,可有效催化前所未有的高度对映选择性的α-氯代酮和取代的α-氯代酮与各种醛的不对称Darzens反应,可直接产生光学活性的二取代和三取代的手性在温和的反应条件下,环氧化物具有较高的产品收率(高达97%)和优异的ee(高达99%)。
更新日期:2017-09-20
中文翻译:

脯氨酸基高效有机催化剂催化的高对映选择性不对称Darzens反应,用于合成二取代和三取代的环氧化物
已发现一类新的易于获得且易于调节的脯氨酸基手性有机催化剂,可有效催化前所未有的高度对映选择性的α-氯代酮和取代的α-氯代酮与各种醛的不对称Darzens反应,可直接产生光学活性的二取代和三取代的手性在温和的反应条件下,环氧化物具有较高的产品收率(高达97%)和优异的ee(高达99%)。



























 京公网安备 11010802027423号
京公网安备 11010802027423号